Rimegepant
- CAS NO.:1289023-67-1
- Empirical Formula: C28H28F2N6O3
- Molecular Weight: 534.56
- MDL number: MFCD23098777
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 14:23:08
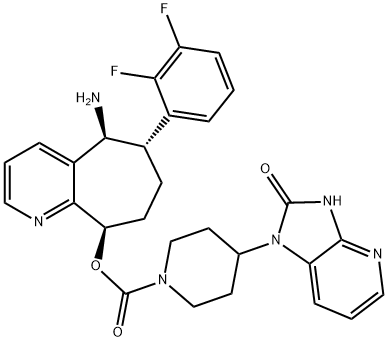
What is Rimegepant?
Absorption
The absolute oral bioavailability of rimegepant is approximately 64%. Following oral administration of the orally disintegrating tablet, maximum plasma concentrations were achieved at 1.5 hours (Tmax).
When administered with a high-fat meal, Tmax is delayed by 1 hour, Cmax is decreased by 42-53%, and AUC is decreased by 32-38%. The clinical significance of this difference in pharmacokinetics is unknown.
Toxicity
There is no specific antidote for rimegepant overdosage and clinical experience is limited. Treatment should consist of general supportive and symptomatic measures including monitoring of vital signs and general observation. Hemodialysis is unlikely to be of benefit given rimegepant's high serum protein binding.
Description
Rimegepant (Nurtec ODT)(FDA approved in February 2020) is an ODT available in a single strength of 75 mg for the acute treatment of migraine with or without aura in adults.No more than 75 mg should be taken in a 24-hour period. while there are no contraindications,concomitant administration with CYP3A4 inhibitors should be avoided.The most common adverse reaction is nausea, occurring in few patients (rimegepant PI, 2020). In clinical trials, a single 75-mg dose of rimegepant demonstrated superiority over placebo with regard to freedom from pain (21% in the rimegepant group vs.11% in the placebo group) and freedom from the most bothersome symptom (35% vs. 27%) at 2 hours post dose (Croop et al., 2019). Rimegepant is not approved for migraine prevention, although it is presently in late-stage clinical trials assessing it for that indication(NCT03732638,2020).
The Uses of Rimegepant
Rimegepant is an oral calcitonin gene-related peptide (CGRP) receptor antagonist used for the treatment of migraine.
Background
Rimegepant is an oral antagonist of the CGRP receptor developed by Biohaven Pharmaceuticals. It received FDA approval on February 27, 2020 for the acute treatment migraine headache, and was subsequently approved by the European Commission in April 2022 for both the treatment and prevention of migraines. While several parenteral antagonists of CGRP and its receptor have been approved for migraine therapy (e.g. erenumab, fremanezumab, galcanezumab), rimegepant and ubrogepant were the only CGRP antagonists that possessed oral bioavailability until the approval of atogepant in 2021.
The current standard of migraine therapy involves abortive treatment with "triptans", such as sumatriptan, but these medications are contraindicated in patients with pre-existing cerebrovascular and cardiovascular disease due to their vasoconstrictive properties. Antagonism of the CGRP pathway has become an attractive target for migraine therapy as, unlike the triptans, oral CGRP antagonists have no observed vasoconstrictive properties and are therefore safer for use in patients with contraindications to standard therapy.
Indications
Rimegepant is indicated for the acute treatment of migraine with or without aura in adults. Rimegepant is also indicated for the prevention of episodic migraine in adults.
Pharmacokinetics
Rimegepant helps to abort migraine headaches by preventing the activity of a pronociceptive molecule that has been implicated in migraine pathophysiology. It is intended for use as an abortive migraine therapy and therefore has a relatively rapid onset of effect, with most efficacy trials evaluating for effect at the 2 hour mark.
Rimegepant does not require dose adjustment in patients with mild, moderate, or severe renal impairment, nor does it require dose adjustment in patients with mild or moderate hepatic impairment. In clinical trials, plasma concentrations of rimegepant were significantly higher in patients with severe (i.e. Child-Pugh C) hepatic impairment - it should therefore be avoided in this population. Hypersensitivity reactions have occurred during clinical studies and patients should be made aware of this possibility. Rimegepant should be discontinued immediately if hypersensitivity reaction occurs.
Metabolism
Rimegepant is metabolized by CYP3A4 and, to a lesser extent, CYP2C9. Specific metabolites of rimegepant have not been characterized and no major metabolites have been detected in plasma. Approximately 77% of an administered dose is eliminated unchanged, suggesting metabolism is likely to be a minor means of drug elimination.
Properties of Rimegepant
| Density | 1.45±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 12.03±0.20(Predicted) |
| form | A crystalline solid |
Safety information for Rimegepant
Computed Descriptors for Rimegepant
Rimegepant manufacturer
LUPA PHARMACEUTICALS
SRINI PHARMACEUTICALS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidYou may like
-
 Rimegepant 98%View Details
Rimegepant 98%View Details -
 1289023-67-1 Rimegepant 98+View Details
1289023-67-1 Rimegepant 98+View Details
1289023-67-1 -
 Rimegepant 98%View Details
Rimegepant 98%View Details -
 Rimegepant API PowderView Details
Rimegepant API PowderView Details
1289023-67-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
