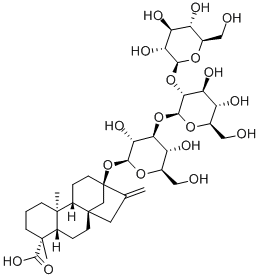
Rebaudioside D
Synonym(s):
- CAS NO.:63279-13-0
- Empirical Formula: C50H80O28
- Molecular Weight: 1129.16
- MDL number: MFCD11113790
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
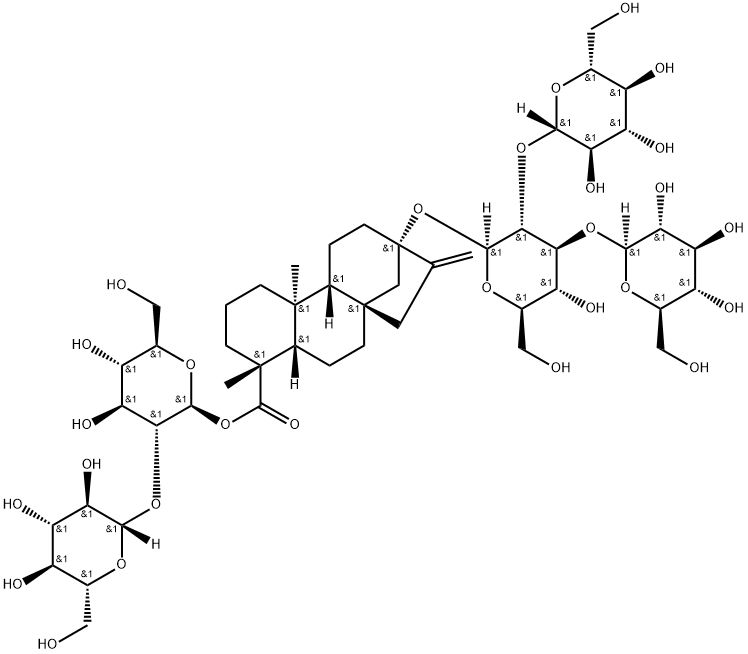
What is Rebaudioside D?
Description
Rebaudioside D, a glycoside of the diterpene steviol is a minor constituent found in leaves of Stevia rebaudiana. It is considered non-caloric and is about 200–220 times sweeter than sucrose.
Rebaudioside D is a natural non-caloric sweetener. It is a steviol glycoside that has been found in S. rebaudiana leaves. Rebaudioside D, similarly to rebaudioside A and rebaudioside C, is metabolized by gut microbiota to steviol, a compound whose safety is widely studied.
The Uses of Rebaudioside D
Rebaudioside D is a steviol glycoside used as a nutritive sweetener and may be used as a replacement for sugar in foods and beverages. Useful in the treatment of blood glucose sensitive diseases such as diabetes, high blood pressure, and obesity.
It may be used as internal standard to optimize the desorption electrospray ionization (DESI) mass spectrometry during the analysis of Stevia leaves for diterpene glycosides.
Preparation
The rebaudioside D-rich steviol glycoside preparation (~95% rebaudioside D) is produced via an enzymatic conversion process using a strain of Pichia pastoris that has been genetically modified to express UDPglucosyltransferase EUGTll. In the first stage of manufacturing, a steviol glycoside primary extract from the leaves of 5. rebaudiana Bertoni containing 55±5% of rebaudioside A is produced according to the methodology outlined in the Chemical and Technical Assessment (CTA) published by the Food and Agriculture Organization (FAO)/JECFA for steviol glycosides (FAQ, 2016). In the next step, the steviol glycoside primary extract is further purified to a purity of ~95% rebaudioside A through crystallization, also consistent with the CTA methodology. In the third stage, the P. pastoris production strain is subjected to a fermentation step to express the UGT-glucosyltransferase EUGTll, which is used to catalyze the conversion of the high-purity rebaudioside A extracted from 5. rebaudiana Bertoni to rebaudioside D. In the last stage, the rebaudioside D solution is purified and concentrated according to the methodology described in the steviol glycoside CTA, yielding a final product that contains ~95% rebaudioside D.
www.fda.gov
Definition
ChEBI: Rebaudioside D is a rebaudioside that is rebaudioside A in which the hydroxy group at position 2 of the beta-D-glucosyl ester moiety has been converted to the corresponding beta-D-glucoside. Found in minute quantities in the leaves of Stevia rebaudiana. It has a role as a sweetening agent. It is a tetracyclic diterpenoid, a rebaudioside and a sophoroside. It is functionally related to a rebaudioside A, a rebaudioside E and a beta-D-Glcp-(1->2)-[beta-D-Glcp-(1->3)]-beta-D-Glcp.
Properties of Rebaudioside D
| Melting point: | >243°C (dec.) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | neat |
| form | Solid |
| color | White to Off-White |
Safety information for Rebaudioside D
Computed Descriptors for Rebaudioside D
| InChIKey | RPYRMTHVSUWHSV-CUZJHZIBSA-N |
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 Rebaudioside D CAS 63279-13-0View Details
Rebaudioside D CAS 63279-13-0View Details
63279-13-0 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4