Quetiapine
- CAS NO.:111974-69-7
- Empirical Formula: C21H25N3O2S
- Molecular Weight: 383.51
- MDL number: MFCD08064204
- EINECS: 601-143-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
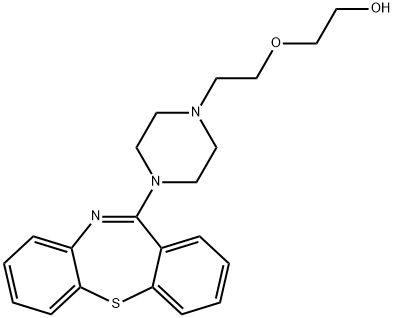
What is Quetiapine?
Absorption
Quetiapine is rapidly and well absorbed after administration of an oral dose. Steady-state is achieved within 48 hours Peak plasma concentrations are achieved within 1.5 hours. The bioavailability of a tablet is 100%. The steady-state Cmax of quetiapine in Han Chinese patients with schizophrenia after a 300 mg oral dose of the extended released formulation was approximately 467 ng/mL and the AUC at steady-state was 5094 ng·h/mL. Absorption of quetiapine is affected by food, with Cmax increased by 25% and AUC increased by 15%.
Toxicity
The oral LD50 if quetiapine in rats is 2000 mg/kg.
Overdose information
Some signs and symptoms of a quetiapine overdose include sedation, drowsiness, tachycardia, and hypotension. Clinical trials demonstrate that overdoses of up to 30 grams of quetiapine did not result in death. A lethal outcome was reported in a clinical trial after an overdose of 13.6 grams of quetiapine. In the case of an acute overdose, ensure to maintain an airway and provide adequate ventilation and oxygenation. Gastric lavage following intubation (if necessary) along with activated charcoal and a laxative may be considered. The possibility of obtundation, seizure or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis. Cardiac monitoring should also take place.
A note on QT-interval prolongation in an overdose
Postmarketing reports reveal increases in the cardiac QT interval in cases of quetiapine overdose, concomitant illness, and in those taking drugs that increase QT interval or affect electrolyte levels. Note that disopyramide, procainamide, and quinidine may exert additive QT-prolonging effects when administered in patients who have overdosed with quetiapine, and should be avoided.
The Uses of Quetiapine
Labelled Quetiapine (Q510000). Used as an antipsychotic.
The Uses of Quetiapine
antiviral
Indications
Quetiapine is used in the symptomatic treatment of schizophrenia. In addition, it may be used for the management of acute manic or mixed episodes in patients with bipolar I disorder, as a monotherapy or combined with other drugs. It may be used to manage depressive episodes in bipolar disorder. In addition to the above indications, quetiapine is used in combination with antidepressant drugs for the treatment of major depression.
Some off-label uses for this drug include the management of post-traumatic stress disorder (PTSD), generalized anxiety disorder, and psychosis associated with Parkinson's disease.
Background
Initially approved by the FDA in 1997, quetiapine is a second-generation atypical antipsychotic used in schizophrenia, major depression, and bipolar disorder. Quetiapine demonstrates a high level of therapeutic efficacy and low risk of adverse effects during long-term treatment. It is well-tolerated and a suitable option for some patients with high sensitivity to other drugs, such as Clozapine and Olanzapine.
Definition
ChEBI: Quetiapine is a dibenzothiazepine, a N-alkylpiperazine and a N-arylpiperazine. It has a role as a serotonergic antagonist, a dopaminergic antagonist, a histamine antagonist, an adrenergic antagonist and a second generation antipsychotic.
brand name
Seroquel (AstraZeneca).
Biological Functions
Quetiapine is a dibenzothiazepine with a brain receptor–binding profile similar to that of clozapine. Quetiapine binds most effectively to histaminergic H1, adrenergic a1 and a2, and serotonergic 5-HT2A receptors in the brain and has even lower affinity than clozapine for dopaminergic D2 receptors. Unlike clozapine, however, quetiapine also has very low affinity for muscarinic receptors.
General Description
Quetiapine, 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]-ethanol fumarate (2:1,salt) (Seroquel), is a white to off-white crystalline powderthat is moderately water soluble. Quetiapine is rapidly absorbed,and peak plasma levels occur 1 to 2 hours after administration.Food does not appreciably affect the absorptionof quetiapine. The compound is 83% bound to plasma proteinsand it has a mean elimination half-life of 7 hours.Administration of a single dose of 14C-quetiapine showedthat only 1% of the drug was excreted unchanged, with 73%excreted into the urine and approximately 30% excreted inthe feces.Numerous metabolites of quetiapine are known,and the sulfoxide metabolite represents the major metabolitepresent in plasma. This metabolite is pharmacologicallyinactive. The remaining metabolites represent only5% of the total radioactivity found in plasma. The 7-hydroxyand the 7-hydroxy-N-desalkyl are active metabolites, but because of their low concentrations in plasma are not thoughtto contribute to the overall effects of quetiapine.
Common side effects associated with quetiapine therapyare orthostatic hypotension and somnolence. These effects are presumably caused by -adrenergic and histamine H1 receptorblockade, respectively. As with other atypical antipsychotics,patients treated with quetiapine should be monitoredfor hyperglycemic symptoms. Also, children and adolescentswith major depressive disorder may experience an increase intheir depression or suicidal tendencies.typical antipsychotics such as chlorpromazine.This findingmay explain the lack of EPS associated with quetiapine.
Pharmacokinetics
Quetiapine improves the positive and negative symptoms of schizophrenia and major depression by acting on various neurotransmitter receptors, such as the serotonin and dopamine receptors. In bipolar disorder, it improves both depressive and manic symptoms.
A note on suicidality in young patients and administration in the elderly
Quetiapine can cause suicidal thinking or behavior in children and adolescents and should not be given to children under 10 years of age. It is important to monitor for suicidality if this drug is given to younger patients. In addition, this drug is not indicated for the treatment of psychosis related to dementia due to an increased death rate in elderly patients taking this drug.
Clinical Use
Schizophrenia
Mania in bipolar disorder
Depression in bipolar disorder
Side Effects
Quetiapine is 100% bioavailable, but first-pass metabolism yields at least 20 metabolites via CYP3A4, with a half-life of approximately 6 hours. Quetiapine is about as effective as haloperidol in treating the positive symptoms of schizophrenia, but it also manages negative symptoms and induces a lower incidence of extrapyramidal side effects.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias.
Antibacterials: concentration possibly increased by
macrolides - avoid.
Antidepressants: concentration of tricyclics possibly
increased.
Antiepileptics: antagonism of convulsive threshold;
metabolism accelerated by carbamazepine and
phenytoin; concentration possibly increased by
valproate.
Antifungals: concentration possibly increased by
imidazoles and triazoles - avoid.
Antimalarials: manufacturer advises avoid use with
artemether and lumefantrine.
Antipsychotics: possible increased risk of ventricular
arrhythmias with risperidone.
Antivirals: concentration possibly increased by
atazanavir, boceprevir, darunavir, fosamprenavir,
indinavir, lopinavir, ritonavir, saquinavir, telaprevir
and tipranavir - avoid.
Anxiolytics and hypnotics: enhanced sedative effects.
Atomoxetine: increased risk of ventricular
arrhythmias.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide.
Grapefruit juice: concentration of quetiapine possibly
increased - avoid.
Metabolism
The metabolism of quetiapine occurs mainly in the liver. Sulfoxidation and oxidation are the main metabolic pathways of this drug. According to in vitro studies, cytochrome P450 3A4 metabolizes quetiapine to an inactive sulfoxide metabolite and also participates in the metabolism of its active metabolite, N-desalkyl quetiapine. CYP2D6 also regulates the metabolism of quetiapine. In one study, three metabolites of N-desalkylquetiapine were identified. Two of the metabolites were identified as N-desalkylquetiapine sulfoxide and 7-hydroxy-N-desalkylquetiapine. CYP2D6 has been found to be responsible for metabolism of quetiapine to 7-hydroxy-N-desalkylquetiapine, a pharmacologically active metabolite. Individual differences in CYP2D6 metabolism may be present, which may affect the concentrations of the active metabolite.
Metabolism
Quetiapine is extensively metabolised in the liver by sulfoxidation mediated mainly by the cytochrome P450 isoenzyme CYP3A4 and by oxidation. The primary metabolite is norquetiapine, which is also eliminated by CYP3A4. Following the administration of radiolabelled quetiapine, the parent compound accounted for less than 5% of unchanged drug-related material in the urine or faeces. Approximately 73% of the radioactivity is excreted in the urine and 21% in the faeces, mainly as inactive metabolites.
Properties of Quetiapine
| Melting point: | 172-173 °C |
| Boiling point: | 556.5±60.0 °C(Predicted) |
| Density | 1.27±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | H2O : < 0.1 mg/mL (insoluble) |
| form | Powder |
| pka | 14.41±0.10(Predicted) |
| color | Light yellow to yellow |
| Stability: | Very Hygroscopic |
| CAS DataBase Reference | 111974-69-7(CAS DataBase Reference) |
Safety information for Quetiapine
Computed Descriptors for Quetiapine
Quetiapine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![DIBENZO[B,F][1,4]THIAZEPINE, 11-OXO-10,11-DIHYDRO- (INTERMEDIATES OF QUETIAPINE)](https://img.chemicalbook.in/CAS/GIF/3159-07-7.gif)
![2-[2-(4-Dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol N-Oxide,Quetiapine N-Oxide](https://img.chemicalbook.in/CAS/GIF/1076199-40-0.gif)
![Quetiapine IMpurity P (11-(4-Ethylpiperazin-1-yl)dibenzo[b,f][1,4]thiazepine fuMarate))](https://img.chemicalbook.in/CAS/20150408/GIF/1011758-03-4.gif)

![dibenzo[b,f][1,4]thiazepin-11-amine](https://img.chemicalbook.in/CAS/GIF/5786-26-5.gif)

You may like
-
 111974-69-7 98%View Details
111974-69-7 98%View Details
111974-69-7 -
 Quetiapine 99%View Details
Quetiapine 99%View Details -
 111974-69-7 Quetiapine 98%View Details
111974-69-7 Quetiapine 98%View Details
111974-69-7 -
 111974-69-7 98%View Details
111974-69-7 98%View Details
111974-69-7 -
 111974-69-7 Quetiapine 99%View Details
111974-69-7 Quetiapine 99%View Details
111974-69-7 -
 Quetiapine 95% CAS 111974-69-7View Details
Quetiapine 95% CAS 111974-69-7View Details
111974-69-7 -
 Quetiapine 98.00% CAS 111974-69-7View Details
Quetiapine 98.00% CAS 111974-69-7View Details
111974-69-7 -
 Quetiapine API PowderView Details
Quetiapine API PowderView Details
111974-69-7
