Potassium benzoate
Synonym(s):Benzoic acid potassium salt
- CAS NO.:582-25-2
- Empirical Formula: C7H5KO2
- Molecular Weight: 160.21
- MDL number: MFCD00013061
- EINECS: 209-481-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
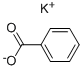
What is Potassium benzoate?
Description
Potassium benzoate is the potassium salt of benzoate. It is mostly used for food preservation for inhibiting the growth of mold, yeast and bacteria since it can create low pH condition after entering into the cells to suppress the anaerobic fermentation of glucose. It can also be used in the whistle in many fireworks. In analytic chemistry, it can be used as eluents for ion chromatography to increase the detector response.
Chemical properties
Potassium benzoate occurs as a slightly hygroscopic, white, odorless or nearly odorless crystalline powder or granules. Aqueous solutions are slightly alkaline and have a sweetish astringent taste.
Chemical properties
Potassium benzoate ( E212 ) , the potassium salt of benzoic acid, is a food preservative that inhibits the growth of mold, yeast and some bacteria. It works best in low-pH products, below 4.5, where it exists as benzoic acid.
Acidic foods and beverages such as fruit juice (citric acid), sparkling drinks (carbonic acid), soft drinks (phosphoric acid), and pickles (vinegar) may be preserved with potassium benzoate. It is approved for use in most countries including Canada, the U.S., and the EU, where it is designated by the E number E212. In the EU, it is not recommended for consumption by children.
The Uses of Potassium benzoate
Pharmaceutic aid (preservative).
The Uses of Potassium benzoate
Potassium Benzoate is manufactured primarily for food and beverage use. It is a chemical preservative, which in very low concentrations inhibits the activity of the microorganisms. It is used in carbonated beverages. The shelf life of un-pasteurized cider can be greatly extended by adding potassium benzoate. It is also used as the whistle in many fireworks.
Production Methods
Potassium benzoate is prepared from the acid–base reaction between benzoic acid and potassium hydroxide.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Potassium benzoate is predominantly used as an antimicrobial
preservative in a wide range of beverages, foods and some
pharmaceutical formulations. Preservative efficacy increases with
decreasing pH; it is most effective at pH 4.5 or below. However, at
low pH undissociated benzoic acid may produce a slight though
discernible taste in food products.
Increasingly, potassium benzoate is used as an alternative to
sodium benzoate in applications where a low sodium content is
desirable.
Therapeutically, potassium benzoate has also been used in the
management of hypokalemia.
Safety Profile
Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Potassium benzoate was recently described by the Food Commission, who campaign for 'safer, healthier food in the UK', as "mildly irritant to the skin, eyes and mucous membranes".
Cats have a significantly lower tolerance to benzoic acid and its salts than rats and mice.
Safety
Potassium benzoate is widely used in food products and is generally
regarded as a nontoxic and nonirritant material. However, people
with a history of allergies may show allergic reactions when exposed
to potassium benzoate. Ingestion is inadvisable for asthmatics.
Higher concentrations of potassium benzoate have been reported to
cause irritation to mucous membranes.
The WHO acceptable daily intake of total benzoates including
potassium benzoate, calculated as benzoic acid, has been estimated
at up to 5 mg/kg of body-weight.
Synthesis
One very common way to make potassium benzoate is by oxidizing toluene.
Another way to synthesize potassium benzoate in the lab setting is by reacting methyl benzoate with potassium thio acetate.
storage
Potassium benzoate is stable at room temperature under normal storage conditions. Since it is slightly hygroscopic, potassium benzoate should be stored in sealed containers. Exposure to conditions of high humidity and elevated temperatures should be avoided.
Purification Methods
Potassium benzoate [582-25-2] M 160.2. Crystallise it from water (1mL/g) between 100o and 0o. [Beilstein 9 III 375, 9 IV 279.]
Mechanism of food preservation
The mechanism of food preservation begins with the absorption of benzoic acid into the cell. If the intracellular pH changes to 5 or lower, the anaerobic fermentation of glucose through phosphofructokinase is decreased by 95 %.
Spectra
Carbon 13 NMR
The carbon NMR shows 5 unique peaks. There are four peaks between 130 - 140 ppm from the carbons in the benzene ring. There is an additional carbon peak around 178 ppm representing the carbon from the carbonyl.
Infrared spectrum The following are the main peaks in the IR spectrum.
1610: C=O from carbonyl
1580: C=C from benzene ring.
Incompatibilities
Potassium benzoate is incompatible with strong acids and strong oxidizing agents.
Regulatory Status
GRAS listed. Accepted as a food additive in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
References
Gjerde, Douglas T., and J. S. Fritz. "Sodium and potassium benzoate and benzoic acid as eluents for ion chromatography." Analytical Chemistry 53.14(1981):2324-2327.
Zengin, N., et al. "The evaluation of the genotoxicity of two food preservatives: Sodium benzoate and potassium benzoate." Food & Chemical Toxicology An International Journal Published for the British Industrial Biological Research Association 49.4(2011):763-9.
Zeb, Alam, et al. "Grape juice preservation with benzoate and sorbate. " Advances in Food Sciences (2009).
Marietta, Michael S. "Fireworks artillery shell." US, US6912958. 2005.
Properties of Potassium benzoate
| Melting point: | >300°C |
| Boiling point: | 450℃[at 101 325 Pa] |
| Density | 1.558 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in ethanol, slightly soluble in methanol, insoluble in ether. |
| form | Solid |
| PH | pH(50g/l, 25℃) : 7.0~9.0 |
| Water Solubility | 492g/L at 20℃ |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Merck | 14,1091 |
| BRN | 3719165 |
| CAS DataBase Reference | 582-25-2(CAS DataBase Reference) |
| EPA Substance Registry System | Benzoic acid, potassium salt (582-25-2) |
Safety information for Potassium benzoate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Potassium benzoate
| InChIKey | XAEFZNCEHLXOMS-UHFFFAOYSA-M |
Potassium benzoate manufacturer
Kronox Lab Sciences Pvt Ltd
Ujwal Pharma Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Potassium benzoate 98%View Details
Potassium benzoate 98%View Details -
 582-25-2 99%View Details
582-25-2 99%View Details
582-25-2 -
 Potassium benzoate 99.00% CAS 582-25-2View Details
Potassium benzoate 99.00% CAS 582-25-2View Details
582-25-2 -
 Potassium benzoate CAS 582-25-2View Details
Potassium benzoate CAS 582-25-2View Details
582-25-2 -
 Potassium benzoate 98%View Details
Potassium benzoate 98%View Details
582-25-2 -
 Potassium benzoate 582-25-2 98%View Details
Potassium benzoate 582-25-2 98%View Details
582-25-2 -
 Potassium benzoate CAS 582-25-2View Details
Potassium benzoate CAS 582-25-2View Details
582-25-2 -
 Potassium benzoate CAS 582-25-2View Details
Potassium benzoate CAS 582-25-2View Details
582-25-2