PLUMBAGIN
Synonym(s):5-Hydroxy-2-methyl-1,4-naphthoquinone
- CAS NO.:481-42-5
- Empirical Formula: C11H8O3
- Molecular Weight: 188.18
- MDL number: MFCD00001682
- EINECS: 207-569-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
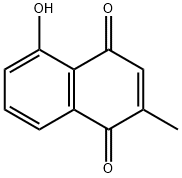
What is PLUMBAGIN?
Description
Plumbagin is a natural 1,4-naphthoquinone first isolated from plants of the genus Plumbago. It has diverse effects in cells and animals. Plumbagin causes the generation of reactive oxygen species and induces apoptosis in cancer cells. It activates signaling through Nrf2 and the antioxidant response element, inducing the expression of Nrf2 target genes, including NQO1 and heme oxygenase-1 in cultured neuronal cells. Plumbagin also inhibits NADPH oxidase 4 in a time- and dose-
Chemical properties
ORANGE CRYSTALLINE POWDER OR CRYSTALS
The Uses of PLUMBAGIN
Plumbagin from Plumbago indica has been used:
- as a reactive oxygen species agent (ROS) to induce cytotoxicity in mouse embryonic fibroblasts
- as an oxidative stress inducer to generate superoxide anion in Saccharomyces cerevisiae
- as a reference standard in thin layer chromatography and in spectrophotometric analysis for quantification of plumbagin in Plumbago auriculate samples
The Uses of PLUMBAGIN
antibacterial, antifungal, tuberculostatic; antifeedant (worm)
What are the applications of Application
Plumbagin is a potent anticancer agent
Definition
ChEBI: A hydroxy-1,4-naphthoquinone that is 1,4-naphthoquinone in which the hydrogens at positions 2 and 5 are substituted by methyl and hydroxy groups, respectively.
General Description
Plumbagin is a bioactive naphthoquinone present majorly in Plumbago indica L. It is a quinoid and is also derived from the roots of Plumbago zeylanica?roots.
Biochem/physiol Actions
Plumbagin exhibits various pharmacological activities including antimicrobial, anticancer, anti-atherosclerotic, antidiabetic, anti-inflammatory, hypolipidemic, and neuroprotective activities. It inhibits the signal transducer and activator of transcription 3 (STAT3) signaling and halts the proliferation of esophageal squamous cell carcinoma (ESCC). Plumbagin elicits protective antioxidative functionality in 4-nitroquinoline-N-oxide (NQNO) induced stress in lymphoma. Plumbagin in a nanoemulsion formulation has antiproliferative effect towards prostate cancer.
in vitro
plumbagin exhibited effective cell growth inhibition via inducing cancer cells to undergo g2/m phase arrest and apoptosis. blockade of cell cycle was associated with increased levels of p21 and reduced amounts of cdc2, cdc25c and cyclinb1. plumbagin treatment also found to enhance the levels of inactivated phosphorylated cdc2 and cdc25c. blockade of p53 activity partially decreased plumbagin-induced apoptosis and g2/m arrest, indicating it might be operated by p53-dependent and independent pathway [1].
in vivo
to determine whether plumbagin inhibited the in vivo tumor growth, a549 cells were injected into nude mice. tumor growth inhibition was most evident in mice treated with plumbagin at 2 mg/kg/day, where around 80% reductions in tumor size were observed, in contrast with mice treated with the vehicle. no sign of toxicity was observed in plumbagin-treated mice as judged by monitoring body weight [1].
Purification Methods
It crystallises in yellow needles from aqueous EtOH. It is soluble in organic solvents, it is steam volatile and it sublimes on heating in a vacuum. [Fieser & Dunn J Am Chem Soc 58 572 1936, Beilstein 8 III 2576, 8 IV 2376.]
References
[1] hsu yl,cho cy,kuo pl,huang yt,lin cc. plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in a549 cells through p53 accumulation via c-jun nh2-terminal kinase-mediated phosphorylation at serine 15 in vitro and in vivo. j pharmacol exp ther.2006 aug;318(2):484-94.
Properties of PLUMBAGIN
| Melting point: | 76-78 °C(lit.) |
| Boiling point: | 283.17°C (rough estimate) |
| Density | 1.354 |
| refractive index | 1.6310 (estimate) |
| storage temp. | -20°C |
| solubility | very faint turbidity in hot Methanol |
| pka | 6.70±0.20(Predicted) |
| form | Crystals or Crystalline Powder |
| color | Orange |
| Merck | 7538 |
| Stability: | Hygroscopic |
| EPA Substance Registry System | 1,4-Naphthalenedione, 5-hydroxy-2-methyl- (481-42-5) |
Safety information for PLUMBAGIN
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for PLUMBAGIN
PLUMBAGIN manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 481-42-5 5-Hydroxy-2-methylnaphthalene-1,4-dione 98%View Details
481-42-5 5-Hydroxy-2-methylnaphthalene-1,4-dione 98%View Details
481-42-5 -
 Plumbagin 98%View Details
Plumbagin 98%View Details -
 Plumbagin 98%View Details
Plumbagin 98%View Details -
 Plumbagin CAS 481-42-5View Details
Plumbagin CAS 481-42-5View Details
481-42-5 -
 Plumbagin 95% CAS 481-42-5View Details
Plumbagin 95% CAS 481-42-5View Details
481-42-5 -
 Plumbagin, from plumbago indica CAS 481-42-5View Details
Plumbagin, from plumbago indica CAS 481-42-5View Details
481-42-5 -
 Plumbagin CAS 481-42-5View Details
Plumbagin CAS 481-42-5View Details
481-42-5 -
 Plumbagin from Plumbago indica CAS 481-42-5View Details
Plumbagin from Plumbago indica CAS 481-42-5View Details
481-42-5
