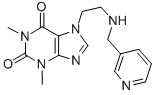
Pimefylline Nicotinate
- Molecular Weight: 0
What is Pimefylline Nicotinate?
Originator
Teonicon, Bracco, Italy ,1975
Manufacturing Process
77 g 7-(β-bromoethyl)-theophylline (C.A. 50, 12071f) and 57.8 g 3picolylamine in 750 ml toluene were refluxed 16 hours with vigorous agitation. The 3-picolylamine hydrobromide formed was filtered off, and the filtrate was evaporated in a vacuum to about one-third of its original volume. About 300 to 400 ml diisopropyl ether were added, and the solution was seeded with a few pure crystals of the desired product.
7-(β-3'-picolylaminoethyl)-theophylline crystallized over a period of a few hours. It was filtered off with suction, washed with a little diisopropyl ether, and dried. The yield of crude product was 69.3 g (82%), its MP 103° to 106°C. The MP was 111° to 112°C after recrystallization from isopropyl acetate. The compound was identified by microanalysis.
39.3 g 7-(β-3'-picolylaminoethyl)-theophylline were dissolved in 300 ml boiling isopropanol, and 15.4 g nicotinic acid were added to the solution in which the acid promptly dissolved. The nicotinate formed crystallized after a short time. It was filtered with suction and dried. The yield was 52.3 g (95.5%). The MP of 159° to 160°C was not significantly changed by recrystallization from ethanol.
Therapeutic Function
Coronary vasodilator
Safety information for Pimefylline Nicotinate
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran
You may like
-
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
![2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%View Details
2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%View Details
740842-50-6 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3