Tetradecafluorohexane
Synonym(s):Perfluorohexane
- CAS NO.:355-42-0
- Empirical Formula: C6F14
- Molecular Weight: 338.04
- MDL number: MFCD00000437
- EINECS: 206-585-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:34
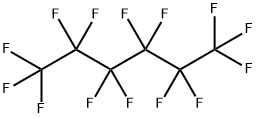
What is Tetradecafluorohexane?
Chemical properties
colourless liquid
The Uses of Tetradecafluorohexane
Perfluorohexanes is used as a coolant, photosensitizer in fluorous biphasic singlet oxygenation and an inert reaction medium. It is used in the electronic cooling liquid and insulator. It plays a vital role as a reaction medium, especially for photooxidation reactions. Further, it finds application as an ultrasound contrast agent.
The Uses of Tetradecafluorohexane
In the electronics industry as a coolant and test bath medium. Non-toxic, non-ozone-depleting, inert reaction medium.
The Uses of Tetradecafluorohexane
Tetradecafluorohexane has been used:
- as a fluorocarbon organic solvent in the preparation of temperature-induced phase-separation solution
- to investigate boiling heat transfer mechanisms
- as a photosensitizer in fluorous biphasic singlet oxygenation
- as a novel reaction medium for photooxidation reactions
What are the applications of Application
Tetradecafluorohexane is A novel reaction medium for photooxidation reactions
What are the applications of Application
Perfluorohexane uses and applications include: dispersant for lubricants, mold release agents, protective coatings; reaction media for polymerizations, purification, separation processes; solvent for removal of halogenated lubricants, oils, greases; carrier for halogenated material; for quick-cooling or freezing foods; heat-transfer fluid.
Because it is biologically inert and chemically stable, perfluorohexane has attracted attention in medicine. Like other fluorocarbons, perfluorohexane dissolves gases, including oxygen from the air, to a higher concentration than ordinary organic solvents. This effect is attributed to the weak intermolecular forces between perfluorohexane molecules, which allows "space" for gas molecules to partition into the liquid. Animals can be submerged in a bath of perfluorohexane without drowning, as there is sufficient oxygen available in the solvent to allow respiration to continue. This effect has led to the experimental use of perfluorohexane in treating burn victims, as their lungs can be filled with either perfluorohexane vapor or in extreme cases liquid perfluorohexane, allowing breathing to continue without the problems normally seen with pulmonary edema that sometimes occur when the inside of the lungs have been burnt e.g. by inhalation of hot smoke.
Definition
ChEBI: Perfluorohexane is a fluoroalkane that is hexane in which all of the hydrogens have been replaced by fluorines. It has a role as a radioopaque medium and a non-polar solvent. It is a fluorocarbon, a fluoroalkane and a volatile organic compound. It derives from a hydride of a hexane.
General Description
Tetradecafluorohexane in the gas phase reacts spontaneously with lithium amalgam, to give a solid and intimate mixture of lithium fluoride and elemental polymeric carbon with a small amount of superstoichiometric lithium.
Metabolism
Not Available
Purification Methods
Purify the fluorohexane by fractional freezing. The methods described for perfluoroheptane should be applicable here. [Beilstein 1 IV 348.]
Properties of Tetradecafluorohexane
| Melting point: | −4 °C(lit.) |
| Boiling point: | 58-60 °C(lit.) |
| Density | 1.669 g/mL at 25 °C(lit.) |
| vapor pressure | 26.5kPa at 25℃ |
| refractive index | n |
| Flash point: | 56-60°C |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 1.699 |
| Water Solubility | Immiscible with water. |
| Merck | 14,7157 |
| BRN | 1802113 |
| Dielectric constant | 1.5700000000000001 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 355-42-0(CAS DataBase Reference) |
| EPA Substance Registry System | Perfluorohexane (355-42-0) |
Safety information for Tetradecafluorohexane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for Tetradecafluorohexane
| InChIKey | ZJIJAJXFLBMLCK-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Perfluoro-compound FC-72* CAS 355-42-0View Details
Perfluoro-compound FC-72* CAS 355-42-0View Details
355-42-0 -
 Perfluorohexane CAS 355-42-0View Details
Perfluorohexane CAS 355-42-0View Details
355-42-0 -
 N-Perfluorohexane CAS 355-42-0View Details
N-Perfluorohexane CAS 355-42-0View Details
355-42-0 -
 Tetradecafluorohexane CAS 355-42-0View Details
Tetradecafluorohexane CAS 355-42-0View Details
355-42-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
