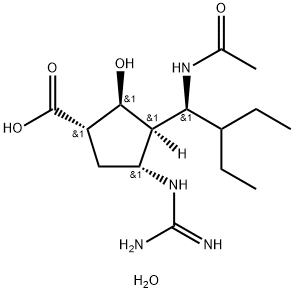
Peramivir
- CAS NO.:330600-85-6
- Empirical Formula: C15H28N4O4
- Molecular Weight: 328.41
- MDL number: MFCD09837902
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-06 15:28:16
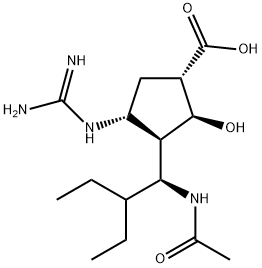
What is Peramivir?
Description
Peramivir is a neuraminidase (NA) inhibitor that was approved in Japan in 2010 for treatment of patients with influenza. It is the only NA inhibitor available for IV use and is the first of two NA inhibitors approved in 2010, the second being the inhaled drug laninamivir octanoate . Peramivir is the only NA inhibitor approved for IV use,which gives it a unique place in influenza treatment for seriously ill patients. Peramivir was discovered using structure-based drug design and is synthesized in six steps from Boc-protected methyl (1S,4R)-4-amino-cyclopent-2-enecarboxylate, which is prepared from 2-azabicyclo[2.2.1]hept-5-en-3-one. Cycloaddition of the cyclopentene olefin with a nitrile oxide provided an intermediate fused cyclopentane-dihydroisoxazole. Hydrogenolysis and acetylation set up a fully functionalized cyclopentane with all four stereocenters established. Deprotection of the amine and acid groups was followed by installation of the guanidine moiety to provide peramivir. Like zanamivir and oseltamivir, peramivir is a potent inhibitor of influenza virus A and B NA [strain A(H1N1) IC50= 0.34 nM; strain A(H3N2) IC50= 0.60 nM; strain B IC50= 1.36 nM]. However, peramivir is less potent against oseltamivirresistant viruses that have the H275Y NA mutation. These viruses remain sensitive to zanamivir. Peramivir is active against influenza A and B viruses and has a lowenzymatic off-rate, suggesting that it could inhibitNAactivity for a prolonged period and allow lower frequency of dosing. Peramivir has proven efficacious in preclinical animal models of influenza infection.
Description
Peramivir is an inhibitor of influenza neuraminidase (IC50s = 0.09 and 11 nM for influenza A and B neuraminidases, respectively). It is selective for influenza neuraminidase over bacterial, mammalian, and other viral neuraminidases (IC50s = >300 μM). Peramivir inhibits neuraminidase activity in H1N1, H2N2, H3N2, and H6N2 influenza strains (IC50s = 0.09-1.1 nM) and reduces lysis of MDCK cells infected with influenza (EC50s = <0.01-21 nM). Pretreatment with peramivir (10-100 mg/kg) protects mice against lethal influenza infections. It also increases survival in ferrets infected intranasally with avian influenza type A H5N1 when injected intramuscularly after infection. Formulations containing peramivir have been used to treat influenza.
Chemical properties
White to Off-White Solid
Originator
BioCryst Pharmaceuticals Inc. (United States)
The Uses of Peramivir
Peramivir is a new antiviral agent for influenza treatment; it can be used as neuraminidase inhibitor for treating human and avian influenza
The Uses of Peramivir
A new antiviral agent for influenza treatment; it can be used as neuraminidase inhibitor for treating human and avian influenza.
Indications
Peramivir is indicated for the treatment of acute uncomplicated influenza in patients six months and older who have been symptomatic for no more than two days.
Background
Peramivir is an antiviral agent developed by Biocryst Pharmaceuticals to treat influenza A/B. The development of peramivir has been supported by the US Department of Health and Human Services as part of the government's effort to prepare for a flu pandemic. Being an influenza virus neuraminidase inhibitor, peramivir works by preventing new viruses from emerging from infected cells. Due to the poor oral bioavailability, the oral formulation of the drug was previously abandoned by Johnson and Johnson Company. The injectable intravenous formulation of peramivir was approved by the FDA in September 2017 for the treatment of acute uncomplicated influenza to pediatric patients 2 years and older who have been symptomatic for no more than two days.
Definition
Peramivir is a member of the class of guanidines that is used (as its trihydrate) for the treatment of acute uncomplicated influenza in patients 18 years and older who have been symptomatic for no more than two days.
brand name
Rapiacta, PeramiFlu
Synthesis
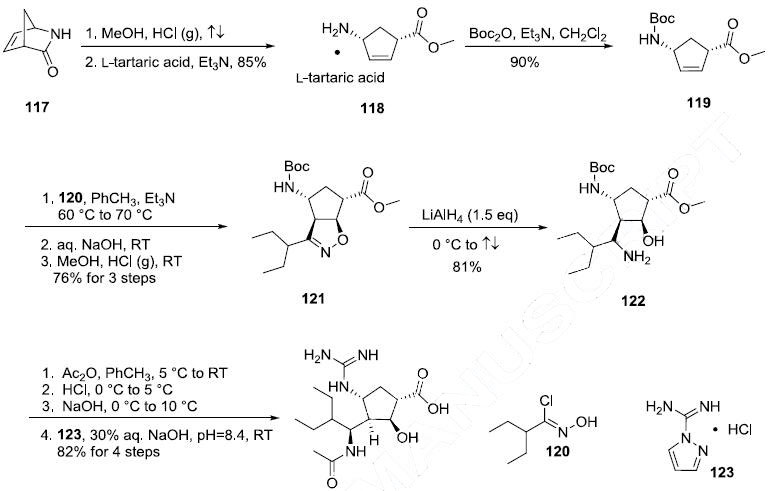
Several syntheses of this drug have been reported and the improved route disclosed in a recent patent is described in the scheme. Ring opening of commercially available (?à)-2- azabicyclo[2.2.1]hept-5-en-3-one (117) with methanolic HCl followed by classical resolution with Ltartaric acid gave the (1S,4R)-methyl ester 118 in 85% yield. Protection of 118 with Boc anhydride and TEA in CH2Cl2 afforded carbamate 119 in 90% yield. Alkene 119 was then subject to nitrone dipolar cycloaddition conditions involving 2-ethyl-N-hydroxybutanimidoyl chloride 120 and triethylamine, followed by the basic workup and then treatment with methanolic HCl, ultimately resulting in dihydroisoxazole 121. Interestingly, the nitrone generated from 120 approached alkene 119 from the less hindered face and proceeded with remarkable regioselectivity to provide azacycle 121 in 76% yield for the three step sequence. Treatment of 121 with 1.5 eq. lithium aluminum hydride resulted in rupture of the N-O bond within this system, which afforded the amino alcohol 122 in 81% yield. It should be noted that neither the Boc group or the methyl ester were reduced under these reaction conditions. Then, a one-pot three step sequence involving acetylation of the amino group, removal of the Boc group, and hydrolysis of the carboxylic ester followed by guanylation with pyrazolecarboxamidine hydrochloride (123) provided peramivir (X) in 82% yield over the final four steps.
Metabolism
Not Available
Properties of Peramivir
| Melting point: | 170 - 172°C (dec.) |
| Density | 1.39 |
| storage temp. | 2-8°C |
| solubility | Methanol (Slightly, Heated), Water (Slightly) |
| form | Solid |
| pka | 4.08±0.70(Predicted) |
| color | White to Off-White |
Safety information for Peramivir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Peramivir
Peramivir manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Peramivir 98%View Details
Peramivir 98%View Details -
 Peramivir 95% CAS 330600-85-6View Details
Peramivir 95% CAS 330600-85-6View Details
330600-85-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8