Pemetrexed
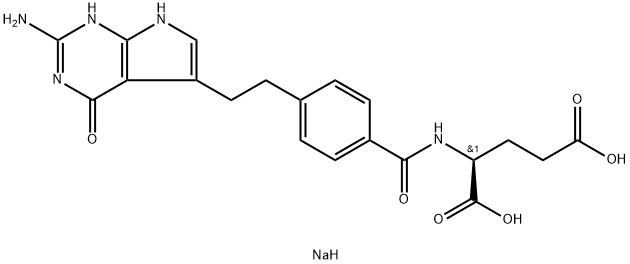
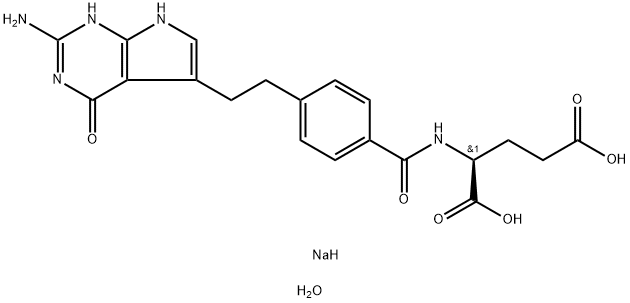
Synonym(s):Pemetrexed disodium heptahydrate
- CAS NO.:137281-23-3
- Empirical Formula: C20H21N5O6
- Molecular Weight: 427.41
- MDL number: MFCD00902635
- EINECS: 680-625-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
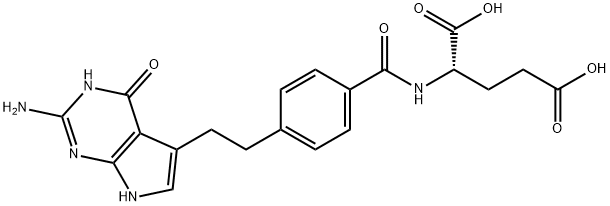
What is Pemetrexed?
Absorption
The pharmacokinetics of pemetrexed when pemetrexed was administered as a single agent in doses ranging from 0.2 to 838 mg/m2 infused over a 10-minute period have been evaluated in 426 cancer patients with a variety of solid tumors. Pemetrexed total systemic exposure (AUC) and maximum plasma concentration (Cmax) increased proportionally with the increase in dose. The pharmacokinetics of pemetrexed did not change over multiple treatment cycles.
Description
Folic acid, or vitamin B9, is essential for many bodily functions, including DNA synthesis, repair, and methylation. In 1941, H. K. Mitchell and co-workers?isolated it by extracting 4 tons of spinach. In 1948, much work on folic acid was published, including the?determination of its structure?by J. H. Mowat and colleagues. Closely related methotrexate is an “antifolate” that can be used to treat cancer, but it has toxic side effects. It is also used to treat some autoimmune diseases and to terminate pregnancy. The more recent, less toxic pemetrexed, another antifolate chemotherapy drug, was approved by the FDA in 2004.
Chemical properties
N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid disodium salt is Off-White Solid
The Uses of Pemetrexed
Pemetrexed is used in the treatment of malignant pleural mesothelioma (MPM) and erectile dysfunction therapy.
Background
Pemetrexed is a chemotherapy drug that is manufactured and marketed by Eli Lilly and Company under the brand name Alimta. It is indicated for use in combination with cisplatin for the treatment of patients with malignant pleural mesothelioma whose disease is either unresectable or who are otherwise not candidates for curative surgery. Its use in non-small cell lung cancer has also been investigated. Pemetrexed was first approved by the FDA in February 4, 2004.
Indications
Pemetrexed is indicated for the treatment of the following conditions:
Non-squamous non-small cell lung cancer (NSCLC)
Malignant pleural mesothelioma
Definition
ChEBI: Pemetrexed is an N-acylglutamic acid in which the N-acyl group is specified as 4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl. Inhibits thymidylate synthase (TS), 421 dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT). It has a role as an antineoplastic agent, an antimetabolite, an EC 2.1.1.45 (thymidylate synthase) inhibitor, an EC 1.5.1.3 (dihydrofolate reductase) inhibitor and an EC 2.1.2.2 (phosphoribosylglycinamide formyltransferase) inhibitor. It is a pyrrolopyrimidine and a N-acyl-L-glutamic acid. It is a conjugate acid of a pemetrexed(2-).
brand name
Alimta (Lilly).
Pharmacokinetics
Pemetrexed inhibited the in vitro growth of mesothelioma cell lines (MSTO-211H, NCI-H2052) and showed synergistic effects when combined with cisplatin. Based on population pharmacodynamic analyses, the depth of the absolute neutrophil counts (ANC) nadir correlates with systemic exposure to pemetrexed and supplementation with folic acid and vitamin B12. There is no cumulative effect of pemetrexed exposure on ANC nadir over multiple treatment cycles.
Side Effects
The most common side effects for pemetrexed include fatigue, nausea, vomiting, anorexia, mucositis, myelosuppression ± infection, bleeding, rash, diarrhea and ↑ LFTs.
Severe myelosuppression is often dose-limiting for pemetrexed. Sepsis, in some cases fatal, have occurred in approximately 1% of patients in clinical trials. Prophylactic folic acid and intramuscular vitamin B12 supplements are necessary to reduce hematologic or non-hematologic toxicities.
Pemetrexed may cause serious and in some cases fatal dermatologic toxicities. Rarely, StevensJohnson syndrome and toxic epidermal necrolysis have been reported.
Metabolism
Pemetrexed is not metabolized to an appreciable extent by the liver.
Storage
Store at +4°C
Mode of action
Pemetrexed is a pyrrolopyrimidine antifolate that exerts its antineoplastic activity by inhibiting thymidylate synthase (TS), glycinamide ribonucleotide formyltransferase (GARFT) and dihydrofolate reductase (DHFR), which are involved in folate metabolism and DNA synthesis, resulting in inhibition of purine and thymidine nucleotide synthesis.
Properties of Pemetrexed
| Density | 1.59±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | slightly sol. in Methanol |
| form | powder to crystal |
| pka | 3.58±0.10(Predicted) |
| color | White to Blue |
| Merck | 14,7077 |
| CAS DataBase Reference | 137281-23-3(CAS DataBase Reference) |
Safety information for Pemetrexed
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pemetrexed
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
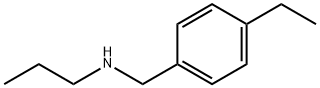
![2-AMINO-5-METHYL-3,7-DIHYDRO-4H-PYRROLO[2,3-D]PYRIMIDIN-4-ONE](https://img.chemicalbook.in/CAS/GIF/62981-82-2.gif)
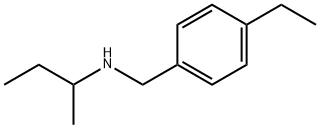
![2-Amino-4-hydroxypyrrolo[2,3-d]pyrimidine](https://img.chemicalbook.in/CAS/GIF/7355-55-7.gif)
![Pyrrolo[2,3-d]pyrimidin-4-ol](https://img.chemicalbook.in/CAS/GIF/3680-71-5.gif)
You may like
-
 137281-23-3 Pemetrexed diacid 98%View Details
137281-23-3 Pemetrexed diacid 98%View Details
137281-23-3 -
 137281-23-3 98%View Details
137281-23-3 98%View Details
137281-23-3 -
 Pemetrexed 99%View Details
Pemetrexed 99%View Details -
 137281-23-3 98%View Details
137281-23-3 98%View Details
137281-23-3 -
 137281-23-3 Pemetrexed 99%View Details
137281-23-3 Pemetrexed 99%View Details
137281-23-3 -
 Pemetrexed CAS 137281-23-3View Details
Pemetrexed CAS 137281-23-3View Details
137281-23-3 -
 Pemetrexed 96% CAS 137281-23-3View Details
Pemetrexed 96% CAS 137281-23-3View Details
137281-23-3 -
 Pemetrexed 95% CAS 137281-23-3View Details
Pemetrexed 95% CAS 137281-23-3View Details
137281-23-3
