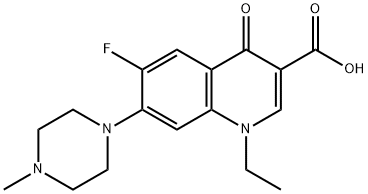
Pefloxacin
Synonym(s):3-Quinolinecarboxylic acid, 1-ethyl-6-fluoro-1,4-dihydro-7-(4-methyl-1-piperazinyl)-4-oxo-, monomethanesulfonate, dihydrate;Pefloxacine monomethanesulfonate dihydrate;Pefloxacinium methanesulfonate dihydrate
- CAS NO.:70458-92-3
- Empirical Formula: C17H20FN3O3
- Molecular Weight: 333.36
- MDL number: MFCD00865015
- EINECS: 274-611-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-21 22:41:43
What is Pefloxacin?
Absorption
Well absorbed by the oral route.
Toxicity
Adverse reactions include peripheral neuropathy, nervousness, agitation, anxiety, and phototoxic events (rash, itching, burning) due to sunlight exposure.
The Uses of Pefloxacin
Pefloxacin Mesylate Dihydrate is an analog of norfloxacin that inhibits topoisomerase.
The Uses of Pefloxacin
antibacterial;DNA gyrase inhibitor
Indications
For the treatment of uncomplicated gonococcal urethritis in males and for gram-negative-bacterial infections in the gastrointestinal system and the genitourinary tract.
Background
A synthetic broad-spectrum fluoroquinolone antibacterial agent active against most gram-negative and gram-positive bacteria.
Definition
ChEBI: A quinolone that is 4-oxo-1,4-dihydroquinoline which is substituted at positions 1, 3, 6 and 7 by ethyl, carboxy, fluorine, and 4-methylpiperazin-1-yl groups, respectively.
Pharmaceutical Applications
A 6-fluoro, 7-piperazinyl quinoline available for oral and intravenous administration. The in-vitro activity is very similar to that of norfloxacin . It is active against H. ducreyi, V. cholerae and Legionella spp., but Campylobacter and Acinetobacter spp. and pseudomonads are not susceptible. It has poor activity against pneumococci, chlamydiae, mycoplasmas and ureaplasmas. L. monocytogenes, Nocardia spp. and anaerobes are resistant.
A plasma concentration of c. 5 mg/L is achieved 1–1.5 h after a 400 mg oral dose. The plasma elimination half-life is 8.5–15 h. It is widely distributed, concentrations in bone, brain, blister fluid, CSF, saliva, sputum and prostate all approximating, and in some cases exceeding, the simultaneous plasma concentration. It is extensively metabolized to the desmethyl (= norfloxacin) and N-oxide derivatives. Some 60–70% of a dose, only about 10% of which is unchanged, appears in the urine; 25% of a dose appears in the feces, a small part contributed by elimination in the bile.
The half-life increases with hepatic impairment, but is virtually unaffected by renal failure. In patients on continuous ambulatory peritoneal dialysis (CAPD) given 800 mg followed by 400 mg every 12 h for 10–12 days, there was no significant accumulation of pefloxacin or its metabolite, norfloxacin, but concentrations of pefloxacin N-oxide rose continuously in plasma and dialysate; all concentrations fell rapidly when treatment was discontinued.
Adverse reactions are those common to the group . Most common are gastrointestinal tract disturbances, although some typical CNS reactions have been encountered. Skin eruptions (some photosensitive) occur and rashes appeared in about one-third of a group of patients who were given longterm therapy. Clinical uses are similar to those of ofloxacin.
Pharmacokinetics
Pefloxacin is a fluoroquinolone antibiotic. Flouroquinolones such as pefloxacin possess excellent activity against gram-negative aerobic bacteria such as E.coli and Neisseria gonorrhoea as well as gram-positive bacteria including S. pneumoniae and Staphylococcus aureus. They also posses effective activity against shigella, salmonella, campylobacter, gonococcal organisms, and multi drug resistant pseudomonas and enterobacter.
Metabolism
Hepatic. Primary metabolites are pefloxacin N-oxide and norfloxacin.
Properties of Pefloxacin
| Melting point: | 270-272° (dec) |
| Boiling point: | 529.1±50.0 °C(Predicted) |
| Density | 1.320±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Aqueous Acid (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Solid |
| pka | 0.16±0.20(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 70458-92-3(CAS DataBase Reference) |
Safety information for Pefloxacin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P321:Specific treatment (see … on this label). P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Pefloxacin
Abamectin manufacturer
Nakoda Chemicals Ltd
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran






You may like
-
 70458-92-3 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid 98%View Details
70458-92-3 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid 98%View Details
70458-92-3 -
 70458-92-3 98%View Details
70458-92-3 98%View Details
70458-92-3 -
 70458-92-3 Pefloxacin 99%View Details
70458-92-3 Pefloxacin 99%View Details
70458-92-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4