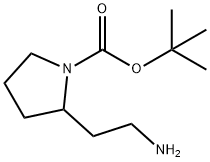
Paxlovid
Synonym(s):(1R,2S,5S)-N-[(1S)-1-Cyano-2-[(3S)-2-oxo-3-pyrrolidinyl]ethyl]-3-[(2S)-3,3-dimethyl-1-oxo-2-[(2,2,2-trifluoroacetyl)amino]butyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide
- CAS NO.:2628280-40-8
- Empirical Formula: C23H32F3N5O4
- Molecular Weight: 499.53
- MDL number: MFCD34473213
- EINECS: 605-001-5
- Update Date: 2024-11-19 15:53:33
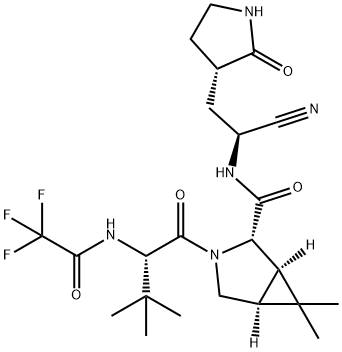
What is Paxlovid?
Absorption
The median Tmax of nirmatrelvir, when given with ritonavir, is 3 hours. After a single oral dose of 300mg nirmatrelvir and 100mg ritonavir in healthy subjects, the Cmax and AUCinf of nirmatrelvir were 2.21 μg/mL and 23.01 μg*hr/mL, respectively.
Toxicity
There are no data regarding overdosage with nirmatrelvir. Treatment of suspected overdose should consist of general supportive measures as clinically indicated.
Description
PF-07321332 is an oral COVID-19 antiviral clinical candidate. By inhibiting the main protease, PF-07321332 prevents the virus from cleaving long protein chains into the parts it needs to reproduce itself.
The Uses of Paxlovid
PF-07321332 (nirmatrelvir) is an oral antiviral clinical lead. It is a covalent inhibitor of SARS-CoV-2 Mpro (main protease, 3CLpro) that binds to cysteine145 within the enzyme's catalytic domain. Mpro is essential for coronavirus replication. Inhibiting Mpro actvity blocks replication at an early stage in the virus' life cycle. Due to structural similarities between the Mpro's of other coronavirusus, PF-07321332 offers the potential of pan-coronavirus activity. In vitro it is suggested to inhibit replication of CoV-2 variants including delta and omicron.
Background
Nirmatrelvir (PF-07321332) is an orally bioavailable 3C-like protease (3CLPRO) inhibitor that is the subject of clinical trial NCT04756531. 3CLPRO is responsible for cleaving polyproteins 1a and 1ab of SARS-CoV-2. Without the activity of the SARS-CoV-2 3CLPRO, nonstructural proteins (including proteases) cannot be released to perform their functions, inhibiting viral replication.
In 2020, Pfizer was investigating another potential treatment for SARS-CoV-2, PF-07304814. Both drugs were inhibitors of SARS-CoV-2 3CLPRO, but nirmatrelvir has the advantage of being orally bioavailable. Nirmatrelvir is advantageous in that it can be prescribed to patients before they require hospitalization, while PF-07304814 requires intravenous administration in hospital.
In December 2021, the FDA granted an emergency use authorization to Paxlovid, a co-packaged product containing both nirmatrelvir and ritonavir, for the treatment of certain patients with mild-to-moderate COVID-19. It was fully approved by the FDA on May 25, 2023. Paxlovid was approved for use in Canada in January 2022 for the treatment of adult patients with mild-moderate COVID-19 and later granted conditional marketing authorization by the European Commission on January 27, 2022.
Indications
In the US, Europe, and Canada, nirmatrelvir, in combination with ritonavir, is indicated for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. In Europe, this therapeutic indication is approved under conditional marketing authorization.
Definition
ChEBI: Nirmatrelvir is an azabicyclohexane that is (1R,5S)-3-azabicyclo[3.1.0]hexane substituted by {(1S)-1-cyano-2-[(3S)-2-oxopyrrolidin-3-yl]ethyl}aminoacyl, 3-methyl-N-(trifluoroacetyl)-L-valinamide, methyl and methyl groups at positions 2S, 3, 6 and 6, respectively. It is the first orally administered inhibitor of SARS-CoV-2 main protease developed by Pfizer and used in combination with ritonavir for the treatment of COVID-19. It has a role as an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor and an anticoronaviral agent. It is a nitrile, a member of pyrrolidin-2-ones, a secondary carboxamide, a pyrrolidinecarboxamide, a tertiary carboxamide, an organofluorine compound and an azabicyclohexane.
Biological Activity
PF-07321332 is an orally bioavailable 3C-like protease (3CLPRO) inhibitor. This drug is being investigated for safety, tolerability, and pharmacokinetics before moving on to studies of efficacy in the treatment or prophylaxis of COVID-19. 3CLPRO is responsible for cleaving polyproteins 1a and 1ab of SARS-CoV-2.
Pharmacokinetics
Nirmatrelvir is rapidly metabolized via CYP3A4 when given alone. In combination with ritonavir, almost no metabolic breakdown of nirmatrelvir takes place, and the drug is excreted primarily through renal elimination. The median Tmax of nirmatrelvir, when given with ritonavir, is 3 hours. After a single oral dose of 300mg nirmatrelvir and 100mg ritonavir in healthy subjects, the Cmax and AUCinf of nirmatrelvir were 2.21 μg/mL and 23.01 μg*hr/mL, respectively.
Mechanism of action
Paxlovid is an active 3Cl protease inhibitor. Paxlovid exerts its antiviral efficacy by inhibiting a necessary protease in the viral replication procedure.
Metabolism
Nirmatrelvir is a substrate of CYP3A4, but undergoes minimal metabolism when administered alongside ritonavir.
Side Effects
People should stop taking Paxlovid and call a health care provider right away if they experience any of the following signs of an allergic reaction: hives; trouble swallowing or breathing; swelling of the mouth, lips, or face; throat tightness; hoarseness; skin rash.
Other possible side effects include an altered or impaired sense of taste, diarrhea, increased blood pressure, muscle aches, abdominal pain, nausea, and feeling generally unwell.
Structure and conformation
PF-07321332 is structurally similar to ML1000. PF-07321332's chemical structure was formally disclosed in Owen et al's Science article in November 2021, and this confirmed the structure that was obtained from the ACS meeting.
Properties of Paxlovid
| Boiling point: | 742.4±60.0 °C(Predicted) |
| Density | 1.267±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : 140 mg/mL (280.26 mM; Need ultrasonic) |
| form | A solid |
| pka | 10.26±0.46(Predicted) |
| color | White to off-white |
Safety information for Paxlovid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Paxlovid
| InChIKey | LIENCHBZNNMNKG-OJFNHCPVSA-N |
| SMILES | [C@@]12([H])[C@@]([H])(C1(C)C)CN(C(=O)[C@@H](NC(C(F)(F)F)=O)C(C)(C)C)[C@@H]2C(N[C@H](C#N)C[C@@H]1CCNC1=O)=O |
Paxlovid manufacturer
Synaptics Labs Private Limited
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran
You may like
-
 2628280-40-8 NIRMALTAVIR 99%View Details
2628280-40-8 NIRMALTAVIR 99%View Details
2628280-40-8 -
 NIRMATRELVIR 98%View Details
NIRMATRELVIR 98%View Details -
 Nirmatrelvir 2628280-40-8 98%View Details
Nirmatrelvir 2628280-40-8 98%View Details
2628280-40-8 -
 2628280-40-8 Nirmatrelvir 99%View Details
2628280-40-8 Nirmatrelvir 99%View Details
2628280-40-8 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3