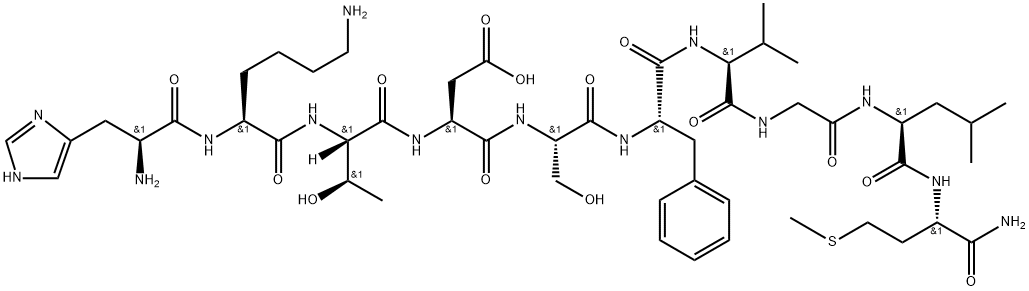
PACAP38
Synonym(s):PACAP 38, Ovine - CAS 137061-48-4 - Calbiochem;PACAP-38;Pituitary Adenylate Cyclase Activating Polypeptide, HSDGIFTDSYSRYRKQMAVKKYLAAVLGKRYKQRVKNK-NH₂
- CAS NO.:137061-48-4
- Empirical Formula: C203H331N63O53S1
- Molecular Weight: 4534.26
- MDL number: MFCD00081800
- Update Date: 2024-11-04 20:04:50
What is PACAP38?
Description
PACAP consists of 27 or 38 aa residues and belongs to the secretin/glucagon superfamily. PACAP has pleiotropic functions, acting as a neurotransmitter and neurotrophic factor in the central nervous system as well as a vasodilator, insulin secretagogue, smooth muscle relaxant, and immunosuppressor in peripheral tissues. PACAP was first isolated in 1989 from the extract of ovine hypothalamus on the basis of its ability to elevate cAMP levels in rat anterior pituitary cells.
The Uses of PACAP38
Pituitary adenylate cyclase activating polypeptide-38 has been used to test its effect in stimulating the formation of cyclic AMP hypothalamus and cerebral cortex slices of chicken and to treat glioblastoma cells (U87MG) in cell migration assay to test its anti-invasive effects.
What are the applications of Application
PACAP(6-38) is a hypothalamic peptide that affects anterior pituitary cell function
General Description
Pituitary adenylate cyclase activating polypeptide-38 (PACAP38) is mapped to human chromosome 18. 27-residue-amidated fragment (PACAP27) comprises another isoform. The PACAP38 is major isoform associated with mammals.
Biological Activity
Endogenous neuropeptide showing considerable homology with vasoactive intestinal peptide (VIP). Potently stimulates adenylyl cyclase.
Biochem/physiol Actions
Pituitary adenylate cyclase activating polypeptide-38 (PACAP38) is a cardioprotectant and may help in treating radiation-induced heart disease (RIHD). It plays a protective role during oxidative stress in cardiomyocytes. PACAP38 has antioxidant, anti-apoptotic and anti-inflammatory property. It is implicated in the pathophysiology of migraine and cluster headache.
storage
Store at -20°C
Structure and conformation
PACAP38, consisting of 38 aa residues, was the first
isolated, followed by PACAP27, lacking 11 aa residues
from the C-terminal of PACAP38. The C-terminal of both
PACAPs was amidated. PACAP belongs to the secretin/
glucagon superfamily, and its closest member is the vasoactive intestinal polypeptide (VIP), which shows 68% aa
sequence identity. Human PACAPs are derived from a
176-aa residue precursor, located in the C-terminus, next
to the PACAP-related peptide (PRP) . The
PACAP precursor is cleaved by various prohormone convertases (PCs) to generate PACAP27 or PACAP38. From
the evolutionary aspect of the superfamily, the ancestral
peptide emerged about 700 million years ago, and
PACAP was established by gene duplication, exon duplication, and exon deletion. The PACAP sequence is well
conserved in vertebrates, and is perfectly conserved in
mammals. Stingray PACAP has 44 aa residues with two predicted processing sites, suggesting that
the processing site of preproPACAP shows diversity in
species. Human PACAP27, theoretical Mr 3147.6, pI 9.70;
human PACAP38, theoretical Mr 4534.3, pI 10.41.
PACAP is freely soluble in water and ethanol. PACAP
solution in water is unstable at 4°C, but is stable for a year
at -80°C at 0.1mM.
Properties of PACAP38
| RTECS | TO7345000 |
| storage temp. | -20°C |
| solubility | H2OPeptide Solubility and Storage Guidelines:1.??Calculate the length of the peptide.2.??Calculate the overall charge of the entire peptide according to the following table:3.??Recommended solution: |
| form | White to off-white solid |
| color | White to off-white |
| Water Solubility | Soluble to 0.90 mg/ml in water |
Safety information for PACAP38
Computed Descriptors for PACAP38
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 PACAP 38, Ovine CAS 137061-48-4View Details
PACAP 38, Ovine CAS 137061-48-4View Details
137061-48-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1