p-Coumaric acid
Synonym(s):trans-4-Hydroxycinnamic acid
- CAS NO.:501-98-4
- Empirical Formula: C9H8O3
- Molecular Weight: 164.16
- MDL number: MFCD00004399
- EINECS: 610-511-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
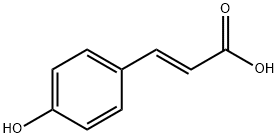
What is p-Coumaric acid?
Description
In 1865, the Austrian chemist H. Hlasiwetz isolated the crystalline solid?p-coumaric acid from plants. The acid is biosynthesized from cinnamic acid and occurs in many parts of legumes and other edible plant species.
Recently, M. R. Berenbaum and colleagues at the University of Illinois (Urbana-Champaign) found that?honeybees fed with honey are more pesticide-resistant?than those fed with substitutes such as corn syrup. They showed that?p-coumaric acid extracted from honey increases bees’ metabolism of the miticide coumaphos.
Chemical properties
Off-white to beige or greenish powder. Soluble in alcohol, ether and hot water, slightly soluble in benzene, insoluble in petroleum ether.
The Uses of p-Coumaric acid
trans-p-Coumaric Acid is the E-isomer of p-Coumaric Acid (C755365), a hydroxy derivative of Cinnamic Acid with antioxidant properties. p-Coumaric acid is a is a major component of lignocellulose. Stud ies suggest that p-Coumaric Acid may reduce the risk of cancer by reducing the formation of carcinogenic nitrosamines.
What are the applications of Application
p-Coumaric acid is a hydroxycinnamic acid with antioxidant properties
Definition
ChEBI: p-coumaric acid is the trans-isomer of 4-coumaric acid. 4-coumaric acid is a coumaric acid in which the hydroxy substituent is located at C-4 of the phenyl ring. It has a role as a plant metabolite. It is a conjugate acid of a 4-coumarate.
What are the applications of Application
trans-4-Hydroxycinnamic acid is used as a hydroxycinnamic acid with antioxidant properties. p-Coumaric acid is an aromatic phytochemical for proteomics research. It has been used as a component of chemiluminescent substrate for protein detection in western blotting.
Preparation
Synthesis of p-Coumaric acid: take o-acetylsalicyloyl chloride as raw material, in the presence of magnesium chloride, react with diethyl malonate in acetonitrile solvent, then add triethylamine, and react at 0°C for 1 h to obtain ( 2-acetoxybenzoyl) diethyl malonate product, then heated to 50°C in potassium hydroxide/methanol solution, reacted for 3h, and cyclized to obtain p-Coumaric acid.
Biochem/physiol Actions
p-Coumaric acid is mainly a plant metabolite which exhibits antioxidant and anti-inflammatory properties. It also shows bactericidal activity by damaging bacterial cell membrane and by interacting with bacterial DNA. In western blot, p-coumaric is also used as an enhancer in chemiluminescent substrate.
Hydroxycinnamic acid found in many fruits and vegetables.
Purification Methods
Crystallise p-coumaric acid from H2O (charcoal). It forms needles from concentrated aqueous solutions as the anhydrous acid, but from hot dilute solutions the monohydrate acid separates on slow cooling. The acid (33g) has been crystallised from 2.5L of H2O (1.5g charcoal) yielding 28.4g of recrystallised acid, m 207o. It is insoluble in *C6H6 or pet ether. The UV in 95% EtOH has max 223 and 286nm ( 14,450 and 19000 M-1cm-1). [UV Wheeler & Covarrubias J Org Chem 28 2015 1963, Corti Helv Chim Acta 32 681 1949, Beilstein 10 IV 1005.]
Properties of p-Coumaric acid
| Melting point: | 214 °C (dec.)(lit.) |
| Boiling point: | 231.61°C (rough estimate) |
| Density | 1.1403 (rough estimate) |
| refractive index | 1.4500 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | ethanol: soluble50mg/mL |
| pka | 4.65±0.10(Predicted) |
| form | Powder |
| color | Off-white to beige or greenish |
| Odor | at 100.00?%. balsamic |
| Water Solubility | soluble |
| Merck | 14,2560 |
| BRN | 2207383 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 501-98-4(CAS DataBase Reference) |
| NIST Chemistry Reference | p-Hydroxycinnamic acid(501-98-4) |
| EPA Substance Registry System | 2-Propenoic acid, 3-(4-hydroxyphenyl)-, (2E)- (501-98-4) |
Safety information for p-Coumaric acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for p-Coumaric acid
| InChIKey | NGSWKAQJJWESNS-ZZXKWVIFSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 501-98-4 trans-4-Hydroxycinnamic acid-98% 99%View Details
501-98-4 trans-4-Hydroxycinnamic acid-98% 99%View Details
501-98-4 -
 trans-4-Hydroxycinnamic acid 98% CAS 501-98-4View Details
trans-4-Hydroxycinnamic acid 98% CAS 501-98-4View Details
501-98-4 -
 p-Coumaric acid, ≥98% (HPLC) CAS 501-98-4View Details
p-Coumaric acid, ≥98% (HPLC) CAS 501-98-4View Details
501-98-4 -
 trans-p-Coumaric Acid CAS 501-98-4View Details
trans-p-Coumaric Acid CAS 501-98-4View Details
501-98-4 -
 p-Coumaric acid CAS 501-98-4View Details
p-Coumaric acid CAS 501-98-4View Details
501-98-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8