OXYCODONE
Synonym(s):(5α)-4,5-Epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one;14-Hydroxydihydrocodeinone;Dihydrone
- CAS NO.:76-42-6
- Empirical Formula: C18H21NO4
- Molecular Weight: 315.36
- MDL number: MFCD00190613
- EINECS: 200-960-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
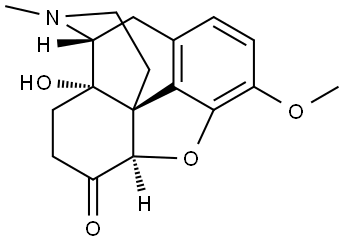
What is OXYCODONE?
Absorption
Oxycodone has an oral bioavailability of 60% to 87% that is unaffected by food.
The area under the curve is 135ng/mL*hr, maximum plasma concentration is 11.5ng/mL, and time to maximum concentration is 5.11hr in patients given a 10mg oral immediate release dose of oxycodone.
Toxicity
Patients experiencing an overdose may present with respiratory depression, sleepiness, stupor, coma, skeletal muscle flaccidity, cold sweat, constricted pupils, bradycardia, hypotension, partial or complete airway obstruction, atypical snoring, and death. Overdose should be treated by maintaining airway, ventilation, and oxygenation. Oxygen and vasopressor treatment may be necessary to treat circulatory shock and pulmonary edema and defibrillation may be required for cardiac arrest of arrhythmia. Naloxone, nalmefene, or naltrexone may be used to counteract the effects of opioids but patients should be monitored in case further doses are required.
The intraperitoneal LD50 in mice is 320mg/kg, the oral LD50 is 426mg/kg. The oral lowest dose causing toxic effects in humans is 0.14mg/kg and subcutaneously in rats it is 1.53mg/kg.
Oxycodone is pregnancy category B according to the FDA. There is a paucity of data regarding oxycodone use in pregnancy, though animal studies show no teratogenic effects. Rats given oxycodone during lactation showed smaller offspring, though after lactation, they recovered to normal size. Oxycodone is excreted in breast milk and so patients should not breastfeed while taking oxycodone due to risk of sedation and respiratory depression in infants.
No studies on the carcinogenicity of oxycodone have been performed. Oxycodone was genotoxic at 50mcg/mL with metabolic activation and at 400mcg/mL without. It was also clastogenic with metabolic activation at ≥1250mcg/mL. Oxycodone was not found to be genotoxic in other tests. Oxycodone does not affect reproduction and fertility in rats at doses of up to 8mg/kg/day.
Description
Oxycodone (Item No. 15465) is an analytical reference material categorized as an opioid. Oxycodone is regulated as a Schedule II compound in the United States. This product is intended for research and forensic applications.
Originator
Oxyfast,Purdue Pharma, L.P.
The Uses of OXYCODONE
Analgesic (narcotic).
Background
Oxycodone is a semisynthetic opioid analgesic derived from thebaine in Germany in 1917. It is currently indicated as an immediate release product for moderate to severe pain and as an extended release product for chronic moderate to severe pain requiring continuous opioid analgesics for an extended period. The first oxycodone containing product, Percodan, was approved by the FDA on April 12, 1950.
Indications
Oxycodone is indicated for the treatment of moderate to severe pain. There is also an extended release formulation indicated for chronic moderate to severe pain requiring continuous opioid analgesics for an extended period.
Definition
oxycodone: An opioid, C18H21N2,similar in structure to codeine butwith a –OH group in codeine replacedby a carbonyl group. It is ananalgesic often used for the treatmentof chronic pain. It is also usedillegally and is a controlled substancein most countries.
brand name
Oxycontin (Purdue); Roxicodone (Roxane); Roxicodone (Xanodyne).
Therapeutic Function
Narcotic analgesic
Biological Functions
Oxycodone is nearly 10 times as strong as codeine, with absorption equal to that of orally administered morphine. Neither hydromorphone nor oxycodone is approved for use in children, and hydromorphone is contraindicated in obstetrical analgesia and in asthmatics.
General Description
Oxycodone is synthesized from the natural opium alkaloidthebaine. Oxycodone is the 14 beta-hydroxyl version of hydrocodone.This additional functional group gives oxycodonegreater potency (1.5 times orally) than hydrocodone presumablyby increasing receptor affinity. The oral bioavailabilityof oxycodone is 65% to 87%. The metabolism of oxycodonefollows the similar pattern of opioid metabolism withN-demethylation, O-demethylation, and their glucuronides allidentified. Per the manufacturer, the analgesic effect of oxycodonecorrelates well with oxycodone plasma concentrations,not the minimal amount of oxymorphone formed, thusoxycodone is not assumed to be a prodrug. There are no largescalestudies of oxycodone used for analgesia in CYP2D6poor metabolizers that can confirm this.
Oxycodone is marketed in combination with acetaminophen(Percocet), aspirin (Percodan), and ibuprofen(Combunox). It has been available for over 50 years as animmediate-release tablet, and in 1995 an extended-releasetablet was approved by the FDA (OxyContin). OxyContin ismanufactured in eight strengths from 10 to 160 mg, and thehigh-dose preparations quickly became attractive to drugabusers. The extended-release tablets are crushed and theninjected or snorted to give an immediate high. The DrugAbuse Warning Network (DAWN) is a public health surveillancesystem that monitors drug-related emergency roomvisits and drug-related deaths. In 1995, they estimated that598,542 emergency room visits involved the nonmedical useof a pharmaceutical (e.g., antidepressant, anxiolytic, stimulant).Of these ER visits, 160,363 visits were attributed toopiates with an estimated 42,810 involving oxycodone or anoxycodone combination. Methadone and hydrocodone/combinationswere estimated to be similar to oxycodone.
Pharmacokinetics
Oxycodone acts directly on a number of tissues not related to its analgesic effect. These tissues include the respiratory centre in the brain stem, the cough centre in the medulla, muscles of the pupils, gastrointestinal tract, cardiovascular system, endocrine system, and immune system. Oxycodone's effect on the respiratory centre is dose dependant respiratory depression. The action on the cough centre is suppression of the cough reflex. Pupils become miopic or decrease in size, peristalsis of the gastrointestinal tract slows, and muscle tone in the colon may increase causing constipation. In the cardiovascular system histamine may be released leading to pruritis, red eyes, flushing, sweating, and decreased blood pressure. Endocrine effects may include increased prolactin, decreased cortisol, and decreased testosterone. It is not yet known if the effects of opioids on the immune system are clinically significant.
Pharmacology
Oxycodone is a potent semisynthetic opioid that has been in use for many years. In addition to actions at the MOP receptor, it may also have analgesic effects mediated via the KOP receptor, resulting in incomplete crosstolerance with morphine. It has a good oral bioavailability, and its plasma concentrations are more predictable than those of morphine after oral administration. It is available in both long- and short-acting oral preparations and, more recently, in a parenteral formulation. Oral oxycodone is roughly 1.5 times more potent than oral morphine.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by subcutaneous route. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Oxycodone's hepatic metabolism is extensive and completed by 4 main reactions. CYP3A4 and 3A5 perform N-demethylation, CYP2D6 performs O-demethylation, unknown enzymes perform 6-keto-reduction, and unknown enzymes perform conjugation.
Oxycodone is metabolized by CYP3A4 and CYP3A5 to noroxycodone and then by CYP2D6 to noroxymorphone. Noroxycodone and noroxymorphone are the primary circulating metabolites. Noroxycodone can also be 6-keto-reduced to alpha or beta noroxycodol.
Oxycodone can be metabolized by CYP2D6 to oxymorphone and then by CYP3A4 to noroxymorphone. Oxymorphone can also be 6-keto-reduced to alpha or beta oxymorphol.
Oxycodone can also be 6-keto-reduced to alpha and beta oxycodol.
The active metabolites noroxycodone, oxymorphone, and noroxymorphone can all be conjugated before elimination.
Properties of OXYCODONE
| Melting point: | 218-220° |
| Boiling point: | 454.92°C (rough estimate) |
| Density | 1.2164 (rough estimate) |
| refractive index | 1.5740 (estimate) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | DMF: 20 mg/ml; DMF:PBS(pH 7.2)(1:1): 0.5 mg/ml; DMSO: 10 mg/ml |
| form | A neat solid |
| pka | 8.53(at 25℃) |
| color | Long rods from EtOH |
| EPA Substance Registry System | Morphinan-6-one, 4,5-epoxy-14-hydroxy-3-methoxy-17-methyl-, (5.alpha.)- (76-42-6) |
Safety information for OXYCODONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects H362:Reproductive toxicity, effects on or via lactation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for OXYCODONE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran







You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1