OPHIOBOLIN A
Synonym(s):
- CAS NO.:4611-05-6
- Empirical Formula: C25H36O4
- Molecular Weight: 400.55
- MDL number: MFCD32182611
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-06 15:14:14
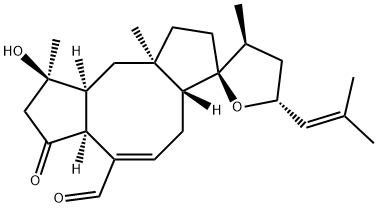
What is OPHIOBOLIN A?
Description
Ophiobolin A (4611-05-6) selectively inhibits the growth of cancer cells (IC50=0.4-4.3 μM) over normal cells (IC50=20.9 μM).1 Induces non-apoptotic cell death in glioblastoma cells and is active in an in vivo model.2 Covalently reacts with primary amines such as lysine side chains2 and phosphatidylethanolamine3 forming unique pyrrole adducts. Induces ER stress4, paraptosis4 and autophagy5.
The Uses of OPHIOBOLIN A
Ophiobolin A is the dominant member of a class of phytotoxic metabolites produced by plant pathogenic fungi. Ophiobolin A acts by inhibiting calmodulin action in calcium regulation.
What are the applications of Application
Ophiobolin A is a sesterterpenoid fungal phytotoxin and inhibitor of calmodulin action in calcium regulation.
Definition
ChEBI: A sesterterpenoid that is ophiobolane with a hydroxy group at position 3, oxo groups at positions 5 and 25, double bonds at positions 7-8 and 19-20, and an oxygen link between positions 14 and 18.
References
1) Bhatia et al. (2016), Anticancer activity of Ophiobolin A, isolated from the endophytic fungus Bipolaris setariae; Nat. Prod. Res., 30 1455 2) Dasari et al. (2015), Fungal metabolite ophiobolin A as a promising anti-glioma agent: In vivo evaluation, structure-activity relationship and unique pyrrolylation of primary amines; Bioorg. Med. Chem. Lett., 25 4544 3) Chidley et al. (2016), The anticancer natural product ophiobolin A induces cytotoxicity by covalent modification of phosphatidylethanolamine; Elife., 5 e14601 4) Kim et al. (2017), Ophiobolin A kills human glioblastoma cells by inducing endoplasmic reticulum stress via disruption of thiol proteostatis; Oncotarget, 8 106740 5) Rodolfo et al. (2016), Ophiobolin A Induces Autophagy and Activates the Mitochondrial Pathway of Apoptosis in Human Melanoma Cells; PLoS One, 11 e0167672
Properties of OPHIOBOLIN A
| Melting point: | 182°C |
| Boiling point: | 443.19°C (rough estimate) |
| Density | 1.0278 (rough estimate) |
| refractive index | 1.4460 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 10 mg/ml) |
| pka | 13.84±0.70(Predicted) |
| form | White to off-white solid. |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
Safety information for OPHIOBOLIN A
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for OPHIOBOLIN A
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Ophiobolin A CAS 4611-05-6View Details
Ophiobolin A CAS 4611-05-6View Details
4611-05-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
