olivetolic acid
- CAS NO.:491-72-5
- Empirical Formula: C12H16O4
- Molecular Weight: 224.25
- MDL number: MFCD28383644
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
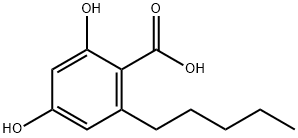
What is olivetolic acid?
The Uses of olivetolic acid
Olivetolic Acid is a precursor in the synthesis of primin and tetrahydrocannabinol.
What are the applications of Application
Olivetolic Acid is a key metabolic intermediate in the biosynthesis of cannabinoids
Definition
ChEBI: A member of the class of benzoic acids that is salicylic acid in which the hydrogens ortho- and para- to the carboxy group are replaced by a pentyl and a hydroxy group, respectively.
Biosynthesis
Olivetolic acid (OLA) biosynthesis involves the condensation of one hexanoyl-CoA with three malonyl-CoAs by a type III PKS (tetraketide synthase) OLA synthase (C. sativa OLS; CsOLS) and an OLA cyclase (CsOAC), both enzymes were derived from the Cannabis species (C. sativa). Studies have achieved trace amount of OLA production in both E. coli and S. cerevisia[1]. Ma et al. find that Pseudomonas sp LvaE encoding a short-chain acyl-CoA synthetase can efficiently convert hexanoic acid to hexanoyl-CoA. The co-expression of the acetyl-CoA carboxylase, the pyruvate dehydrogenase bypass, the NADPH-generating malic enzyme, as well as the activation of peroxisomal β-oxidation pathway and ATP export pathway are effective strategies to redirect carbon flux toward OLA synthesis.
References
[1] Ma, J, Y. Gu, and P. Xu . "Biosynthesis of cannabinoid precursor olivetolic acid by overcoming rate-limiting steps in genetically engineered Yarrowia lipolytica." Cold Spring Harbor Laboratory(2021).
Properties of olivetolic acid
| Melting point: | 143 °C |
| Boiling point: | 403.9±25.0 °C(Predicted) |
| Density | 1.237±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 3.41±0.34(Predicted) |
| color | Light Beige to Light Brown |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C12H16O4/c1-2-3-4-5-8-6-9(13)7-10(14)11(8)12(15)16/h6-7,13-14H,2-5H2,1H3,(H,15,16) |
Safety information for olivetolic acid
Computed Descriptors for olivetolic acid
| InChIKey | SXFKFRRXJUJGSS-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C1=C(CCCCC)C=C(O)C=C1O |
olivetolic acid manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateYou may like
-
 491-72-5 Olivetolic acid 98%View Details
491-72-5 Olivetolic acid 98%View Details
491-72-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1