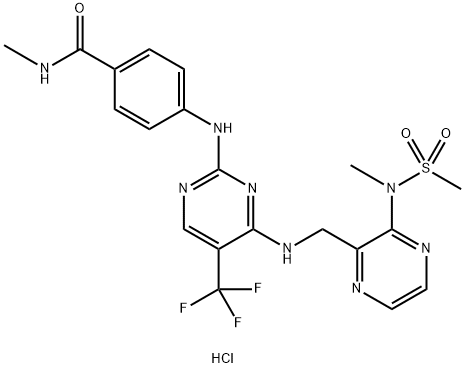
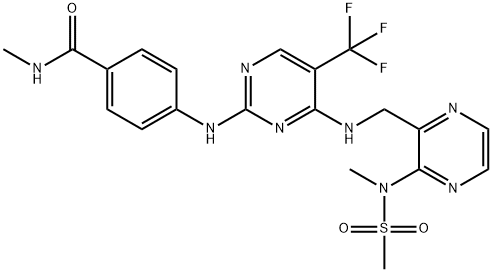
ODM-201
- CAS NO.:1297538-32-9
- Empirical Formula: C19H19ClN6O2
- Molecular Weight: 398.85
- MDL number: MFCD29472270
- EINECS: 829-313-8
- Update Date: 2024-12-25 18:09:02
What is ODM-201?
Absorption
Darolutamide is absorbed in the gastrointestinal tract. In the fasted state, peak concentrations are reached within 3-5 hours, and within 3-8 hours in the fed state. Median Tmax is between 3-6 hours.The average darolutamide steady-state peak plasma concentration after a 600 mg twice daily dose is approximately 4.79 mg/L. The Cmax is attained approximately 4 hours after administration of a single 600 mg oral dose. The AUC 0-12h is approximately 52.82 h?μg/mL.
Effects of food
The absolute bioavailability of darolutamide is approximately 30% after fasting and taking a single 300 mg dose. Steady-state concentrations are attained between 2 and 5 days after repeated administration with food. The bioavailability of darolutamide increases by 2.0 to 2.5 times when it is given with food.
Toxicity
LD50 information for darolutamide is not readily available in the literature.
To this date, there is no known antidote in existence for an overdose with darolutamide. The highest dose clinically documented was a twice daily dose of 900 mg, totalling 1800 mg. Dose-limiting toxicities have not been observed with this drug. In patients with healthy kidney and liver function, a high dose of darolutamide will likely not lead to systemic toxicity. If a high dose (higher than recommended on labeling) is ingested in a patient with renal or hepatic impairment, and toxic symptoms occur, pause treatment with darolutamide and offer supportive treatment until symptoms resolve.
Description
ODM-201 is a novel androgen receptor (AR) inhibit designed to inhibit the growth of prostate cancer cells through binding to the AR to inhibit the testosterone-induced AR nuclear translocation. It is particularly used in patients with progressive metastatic castration-resistant prostate cancer. It is effective in inhibiting the activity of some mutant ARs emerging during antiandrogen therapies, including the F876L mutation version which is resistant to enzalutamide and ARN-509 (the second-generation antiandrogens).Therefore, it has potential to overcome the resistance issue occurring upon AR-targeted therapies.
Description
ODM-201 is an androgen receptor (AR) antagonist (Ki = 11 nM). It inhibits AR transactivation in U2OS osteosarcoma cells expressing human wild-type and mutant ARs (IC50s = 65, 66, 1,500, and 1,782 nM for wild-type, ARF876L, ARW741L, and ART877A, respectively). ODM-201 prevents androgen-induced AR nuclear translocation in AR-overexpressing HS-HEK293 and LNCaP cells and suppresses androgen-induced proliferation of VCaP cells (IC50 = 230 nM). In vivo, ODM-201 (50 mg/kg per day) reduces tumor growth in a VCaP castrated mouse xenograft model. It also inhibits tumor growth without increasing serum testosterone levels in a VCaP intact mouse xenograft model.
The Uses of ODM-201
ODM-201 is a compound being used as a novel next-generation androgen receptor-directed therapy for prostate cancer.
Indications
Darolutamide is indicated for the treatment of adults with non-metastatic castration-resistant prostate cancer (nmCRPC) and metastatic hormone-sensitive prostate cancer (mHSPC) in combination with docetaxel.
Background
Darolutamide is a nonsteroidal androgen receptor antagonist for the treatment of castrate-resistant, non-metastatic prostate cancer (nmCRPC). This condition occurs in the majority of patients with advanced prostate cancer who have been treated with androgen receptor antagonists. Though prior treatment for prostate cancer has been successful for these patients, the cancer eventually progresses to become resistant to existing therapies. This warrants further treatment.
The goal of treatment with darolutamide is to delay the progression of prostate cancer to metastatic disease, increasing quality of life and life expectancy for those with advanced prostate cancer. Darolutamide was developed by Bayer HealthCare Pharmaceuticals Inc. and approved by the FDA on July 30th, 2019.
Pharmacokinetics
Darolutamide, through its downstream effects on cancer cell growth, treats castrate-resistant prostate cancer. It inhibits cancer cell growth and markedly lowers prostate specific antigen (PSA) levels through potent androgen receptor antagonism.
Metabolism
Darolutamide is mainly metabolized by the CYP3A4 hepatic microsomal enzyme in addition to UGT1A9 and UGT1A1. The main active metabolite keto-darolutamide in found in the plasma at 2 times the concentration of darolutamide.
References
Moilanen, Anu Maarit, et al. "Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies." Sci Rep 5.2(2015):12007. Fizazi, Karim, et al. "ODM-201, a new generation androgen receptor inhibitor for castration-resistant prostate cancer: Preclinical and phase I data." Genitourinary Cancers Symposium of the Conquer-Cancer-Foundation of 2013. Fizazi, K, et al. "Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial." Lancet Oncology 5.9(2014):975-85.
Properties of ODM-201
| Melting point: | 169 - 171°C |
| Boiling point: | 719.5±60.0 °C(Predicted) |
| Density | 1.41±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 11.10±0.10(Predicted) |
| color | White to Off-White |
Safety information for ODM-201
Computed Descriptors for ODM-201
ODM-201 manufacturer
Venkatasai Life Sciences
Vijaya Pharma And Life Science
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 1297538-32-9 Darolutamide 99%View Details
1297538-32-9 Darolutamide 99%View Details
1297538-32-9 -
 1297538-32-9 98%View Details
1297538-32-9 98%View Details
1297538-32-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8