Octopamine
- CAS NO.:104-14-3
- Empirical Formula: C8H11NO2
- Molecular Weight: 153.18
- MDL number: MFCD00044589
- EINECS: 203-179-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-28 13:53:27
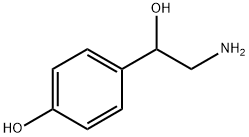
What is Octopamine?
Description
An alkaloid found in annual rye grass, this base has been suggested as a possible precursor of annuloline (q.v.).
Originator
Norfen,Morishita, Japan ,1975
The Uses of Octopamine
Octopamine is a biogenic monoamine structurally related to Noradrenaline (N661038), it acts as a neurohormone, neuromodulator and a neurotransmitter in invertebrates.
Definition
ChEBI: Octopamine is a member of the class of phenylethanolamines that is phenol which is substituted at the para- position by a 2-amino-1-hydroxyethyl group. A biogenic phenylethanolamine which has been found to act as a neurotransmitter, neurohormone or neuromodulator in invertebrates. It has a role as a neurotransmitter. It is a member of phenylethanolamines and a member of tyramines. It is a conjugate base of an octopaminium.
Manufacturing Process
A solution of 33 grams of anhydrous aluminum chloride in 60 grams of nitrobenzene, to which a mixture of 14 grams of phenol and 9.3 grams of hydrochloride of amino-acetonitrile was added, had dry hydrochloric acid gas introduced into it for 3 hours, while stirring and cooling to keep the temperature between 20° and 30°C. The reaction mixture was then poured, with cooling, into 70 cc of water and the deposit obtained was sucked off, washed with acetone and dissolved in 300 cc of water. The solution thus prepared was decolorized with carbon, 50 grams of 30% sodium citrate solution was added to it, and then it was made slightly alkaline with ammonia. Thereupon hydroxy-4'-phenyl-1-amino-2-ethanone crystallized out in the form of leaflets. The yield was 7.7 grams.
The hydrochloride of this base, obtained by evaporation to dryness of a solution of the base in dilute hydrochloric acid and subsequent treatment of the residue with ethyl alcohol and acetone, had a chlorine content of 18.84%, (calculated, 18.90%).
This hydrochloride, on being dissolved in water and hydrogenated with hydrogen and a nickel catalyst, gave a good yield of hydrochloride of hydroxy4'-phenyl-1-amino-2-ethanol melting, after crystallization from a mixture of ethyl alcohol and butanone-2, at from 177° to 179°C with decomposition
Therapeutic Function
Hypertensive
References
Hardwick, Axelrod, Plant Physiol., 44, 1745 (1969)
Properties of Octopamine
| Melting point: | 157-158 °C |
| Boiling point: | 276.1°C (rough estimate) |
| Density | 1.1577 (rough estimate) |
| refractive index | 1.5010 (estimate) |
| pka | pKa 8.88 (Uncertain);9.53 (Uncertain) |
| CAS DataBase Reference | 104-14-3(CAS DataBase Reference) |
Safety information for Octopamine
Computed Descriptors for Octopamine
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![(1R)-2-amino-1-[4-(trifluoromethoxy)phenyl]ethanol](https://img.chemicalbook.in/CAS/20180527/GIF/1568041-73-5.gif)
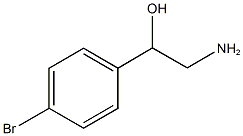
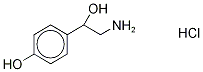
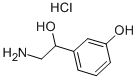
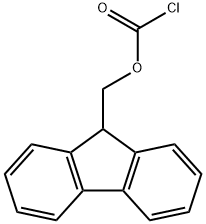
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1