Octadecanoic acid, ester with 1,2,3-propanetriol hexadecanoate
- CAS NO.:8067-32-1
- Empirical Formula: C37H76O7
- Molecular Weight: 632.99514
- MDL number: MFCD29767828
- EINECS: 2325148
- Update Date: 2023-05-25 18:01:01
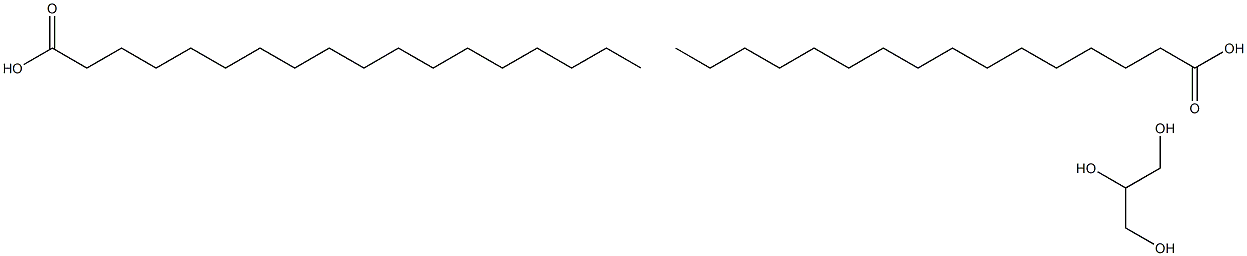
What is Octadecanoic acid, ester with 1,2,3-propanetriol hexadecanoate ?
Chemical properties
Glyceryl palmitostearate occurs as a fine white powder with a faint odor.
Production Methods
Glyceryl palmitostearate is manufactured, without a catalyst, by the direct esterification of palmitic and stearic acids with glycerol.
Pharmaceutical Applications
Glyceryl palmitostearate is used in oral solid-dosage pharmaceutical
formulations as a lubricant. Disintegration times increase and
tablet strength decreases with increase in mixing time.
It is used as a lipophilic matrix for sustained-release tablet and
capsule formulations. Tablet formulations may be prepared by
either granulation or a hot-melt technique, the former producing
tablets that have the faster release profile. Release rate decreases
with increased glyceryl palmitostearate content.
Glyceryl palmitostearate is used to form microspheres, which
may be used in capsules or compressed to form tablets,
pellets, coated beads, and biodegradable gels.
Safety
Glyceryl palmitostearate is used in oral pharmaceutical formulations
and is generally regarded as an essentially nontoxic and
nonirritant material.
LD50 (rat, oral): >6 g/kg
storage
Glyceryl palmitostearate should not be stored at temperatures above 35°C. For storage for periods over 1 month, glyceryl palmitostearate should be stored at a temperature of 5–15°C in an airtight container, protected from light and moisture.
Incompatibilities
Glyceryl palmitostearate is incompatible with ketoprofen(15) and naproxen.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (oral suspension, oral tablet). Included in nonparenteral preparations licensed in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Octadecanoic acid, ester with 1,2,3-propanetriol hexadecanoate
| solubility | Freely soluble in chloroform and dichloromethane;
practically insoluble in ethanol (95%), mineral oil, and water. |
Safety information for Octadecanoic acid, ester with 1,2,3-propanetriol hexadecanoate
Computed Descriptors for Octadecanoic acid, ester with 1,2,3-propanetriol hexadecanoate
Octadecanoic acid, ester with 1,2,3-propanetriol hexadecanoate manufacturer
Subhash Chemical Industries Private Limited
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 8067-32-1 Glyceryl palmitostearate 98%View Details
8067-32-1 Glyceryl palmitostearate 98%View Details
8067-32-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8

