OCTACHLOROSTYRENE
- CAS NO.:29082-74-4
- Empirical Formula: C8Cl8
- Molecular Weight: 379.71
- MDL number: MFCD00661058
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
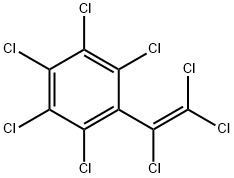
What is OCTACHLOROSTYRENE?
Description
Octachlorostyrene(OCS) is a persistent, highly bioaccumulative, and toxic halogenated aromatic compound. It is not commercially manufactured, but is reportedly an inadvertent by-product of processes that combine carbon and chlorine under elevated temperatures. Magnesium production, chloride solvent production, aluminum plasma etching, aluminum degassing with hexachloroethane, chlorination of titanium, waste incineration, and chloro-alkali production with graphite anodes process are considered to be candidate occupations that produce OCS. However, recent advances have been made in process technology and pollution prevention practices in some of these industries, such as largely eliminating the electrolytic manufacture of chlorine and aluminum degassing with hexachloroethane, both of which have likely resulted in reductions in known sources of OCS.
The Uses of OCTACHLOROSTYRENE
OCS is used in pesticide products to increase the effectiveness of the active ingredients, to make the product easier to apply, or to allow several active ingredients to mix in one solution.
The Uses of OCTACHLOROSTYRENE
Perchlorostyrene is a component used in pesticide formulations.
Definition
ChEBI: Octachlorostyrene is an organochlorine compound.
Safety Profile
Moderately toxic by ingestion.When heated to decomposition it emits toxic vapors ofClí.
Environmental Fate
OCS is bioaccumulative in aquatic food webs. Due to its low
water solubility (water solubility = 1.74E-03 mg l-1; log P
(octanol–water) = 7.460), OCS tends to rapidly partition from
water and binds to sediments and suspended solids. Bioconcentration
through direct uptake may be an important
mechanism in aquatic species.
In aquatic systems, OCS is expected to adsorb to suspended
solids and sediments based on its Koc value ranging from
200 000 to 10 000 000. OCS has been detected in water at
concentrations as high as 7.2 ng l-1, but levels typically are well
below 1 ng l-1. While there is the potential for volatilization
from aquatic systems based on an estimated Henry’s law
constant of 2.3 ×10-4 atm m3 mol-1, volatilization (vapor
pressure= 1.32E-05mmHg) is likely attenuated by adsorption
to particles. Bioaccumulation by aquatic organisms is likely
based on a bioconcentration factor that is estimated to range
from 8100 to 33 000. Field estimates of bioaccumulation factors range up to 1 400 000 (from water to rainbow trout in
Lake Ontario). Mean concentrations in Lake Ontario sediments
and rainbow trout were 13.6 ng g-1 dry weight (ppb) and
2.6 ng g-1 wet weight (ppb), respectively. The highest
concentrations found in fish as part of the National Study of
Chemical Residues in Fish (conducted by the US Environmental
Protection Agency (EPA)) were from Bayou D’Inde,
Louisiana (138 ng g-1 (ppb)), Freeport, Texas (65.3 ng g-1
(ppb)), River Rouge, Michigan (50.7 ng g-1 (ppb)), and Olcott,
New York (49.6 ng g-1 (ppb)). Temporal studies, while
limited, have indicated a substantial decline in concentrations
of OCS since the 1970s. In contrast, relatively low OCS levels
in freshwater mussels and fish from Belgium and Romania
ranged from 0.01 to 0.18 ng g-1 wet weight (ppb), and those in
marine fish (bib, sole, and whiting) ranged from 0.01 to
0.02 ng g-1 wet weight (ppb).
In terrestrial systems, OCS is expected to bind to soil
particles. In the atmosphere, OCS (in the vapor phase) is
degraded by reactions with photochemically produced
hydroxyl radicals. OCS weakly absorbs ultraviolet light
between 295 and 310 nm with slow photolysis. Major transformation
products of photolysis include heptachlorostyrene
and two isomers of hexachlorostyrene, while minor transformation
products of photolysis include pentachlorostyrene
and tetrachlorostyrene.
Monitoring of OCS in western Hudson polar bears showed
no change during 1991–2007. This suggests the persistency of
OCS in the environment, though it did not further accumulate.
Toxicity evaluation
The mechanisms of toxicity and the human toxicological properties of OCS have not been well characterized. Exposure to OCS decreased GSH, increased reactive oxygen species and cytosolic caspase-3 activation in human Chang liver cells, and led to cell death. These results suggest that the toxicity in cells may be via apoptotic processes.
Properties of OCTACHLOROSTYRENE
| Melting point: | 92.5-96.5 °C |
| Boiling point: | 464.98°C (rough estimate) |
| Density | 1.9104 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| Flash point: | 2 °C |
| storage temp. | APPROX 4°C
|
| solubility | Chloroform (Slightly), Methanol (Very Slightly) |
| form | neat |
| EPA Substance Registry System | Octachlorostyrene (29082-74-4) |
Safety information for OCTACHLOROSTYRENE
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for OCTACHLOROSTYRENE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid N-octanoyl benzotriazole 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1