OCHRATOXIN B
- CAS NO.:4825-86-9
- Empirical Formula: C20H19NO6
- Molecular Weight: 369.37
- MDL number: MFCD00133680
- EINECS: 621-766-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 21:30:20
What is OCHRATOXIN B?
Description
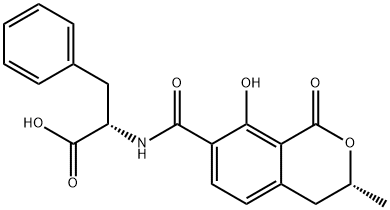
Ochratoxins are mycotoxins produced by Aspergillus and Penicillium species of fungi that contaminate foods. Ochratoxin A (OTA, ) is a chlorinated form with toxicity that targets the kidneys, causing nephropathy and renal adenomas. OTB is a non-
The Uses of OCHRATOXIN B
Ochratoxins are toxic metabolites from Aspergillus orchraceus.
The Uses of OCHRATOXIN B
Ochratoxin B is the non-chlorinated analogue of the much more extensively studied ochratoxin A. It is co-produced by the same species of Aspergillus and Penicillium that are associated with food spoilage. Ochratoxin B has received little focused investigation and its mode of action and potential hazards have been inferred from ochratoxin A.
Definition
ChEBI: Ochratoxin B is a phenylalanine derivative resulting from the formal condensation of the amino group of L-phenylalanine with the carboxy group of (3R)-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-2-benzopyran-7-carboxylic acid. Ochratoxin B differs from the more naturally abundant ochratoxin A in the absence of the dihydroisocoumarin chlorine atom. It has cytotoxic effects on kidney and liver cells in vitro but only minor effects in vivo, due to its rapid metabolism and excretion. It inhibits cell proliferation of human liver HepG2 cells at doses as low as 1 mug/ ml but lacks the genotoxic activity of ochratoxin A, even at higher concentrations. It has a role as an Aspergillus metabolite, a Penicillium metabolite, a mycotoxin and a calcium channel blocker. It is a phenylalanine derivative, a N-acyl-L-phenylalanine, a member of isochromanes and a monocarboxylic acid. It is a conjugate acid of an ochratoxin B(1-).
What are the applications of Application
Ochratoxin B is the non-chlorinated and less potent analog of Ochratoxin A
General Description
Crystals that exhibit blue fluorescence.
Reactivity Profile
OCHRATOXIN B is incompatible with strong oxidizing agents, strong acids and strong bases. OCHRATOXIN B is a carboxylic acid derivative. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt.
Fire Hazard
Flash point data for OCHRATOXIN B are not available. OCHRATOXIN B is probably combustible.
Properties of OCHRATOXIN B
| Melting point: | 221° (van der Merwe) |
| Boiling point: | 632.4±55.0 °C(Predicted) |
| alpha | D -35° (c = 0.15 in ethanol) |
| Density | 1.361±0.06 g/cm3(Predicted) |
| Flash point: | -11 °C |
| storage temp. | 2-8°C |
| solubility | DMF: 10 mg/ml; DMSO: 15 mg/ml; Ethanol: 50 mg/ml; Ethanol:PBS (pH 7.2) (1:1): 0.50 mg/ml |
| form | A crystalline solid |
| pka | 3.40±0.10(Predicted) |
| color | White to off-white |
| EPA Substance Registry System | L-Phenylalanine, N-[[(3R)-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl]carbonyl]- (4825-86-9) |
Safety information for OCHRATOXIN B
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H312:Acute toxicity,dermal H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for OCHRATOXIN B
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





![OCHRATOXIN, B-, [3H(G)]](https://img.chemicalbook.in/StructureFile/ChemBookStructure6/GIF/CB3321324.gif)
You may like
-
 Ochratoxin B solution CAS 4825-86-9View Details
Ochratoxin B solution CAS 4825-86-9View Details
4825-86-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
