NPGB
Synonym(s):4-Guanidinobenzoic acid 4-nitrophenylester hydrochloride;NPGB;pNPGB
- CAS NO.:19135-17-2
- Empirical Formula: C14H13ClN4O4
- Molecular Weight: 336.73
- MDL number: MFCD00037010
- EINECS: 242-831-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:13
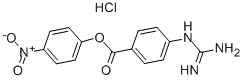
What is NPGB?
The Uses of NPGB
4-Nitrophenyl 4-guanidinobenzoate hydrochloride has been used:
- as a substrate for trypsin for active site titration experiments
- for pre-treating of mosquito eggs in the interplasmid transposition assay
- as a component of isotonic buffer to moisten filter paper for mosquito embryo collection
General Description
4-Nitrophenyl 4-guanidinobenzoate hydrochloride (pNPGB) is a trypsin substrate. It is more water-soluble than 4-methylumbelliferyl 4-guanidinobenzoate and is preferred in spectrophotometric assay.
Biochem/physiol Actions
4-Nitrophenyl 4-guanidinobenzoate hydrochloride (pNPGB) inhibits phenoloxidase enzyme and delays embryo chorionic membrane hardening.
References
[1] BHUMI PATEL . L. donovani XPRT: Molecular characterization and evaluation of inhibitors[J]. Biochimica et biophysica acta. Proteins and proteomics, 2018, 1866 3: Pages 426-441. DOI:10.1016/j.bbapap.2017.12.002.
[2] FRANK S. STEVEN Margaret M G. Studies on the Molecular Mechanism of Mersalyl and 4-Aminophenylmercuric Acetate Re-activation of Trypsin-Thiol Complexes[J]. The FEBS journal, 1980, 109 2: 567-573. DOI:10.1111/j.1432-1033.1980.tb04829.x.
[3] YING-YING HE . Isolation, expression and characterization of a novel dual serine protease inhibitor, OH-TCI, from king cobra venom[J]. Peptides, 2008, 29 10: Pages 1692-1699. DOI:10.1016/j.peptides.2008.05.025.
[4] J.A. SCHIFFERLI G. S. A simple two-step procedure for the preparation of the first component of human complement (C1) in its native form[J]. Journal of immunological methods, 1985, 76 2: Pages 283-288. DOI:10.1016/0022-1759(85)90305-9.
[5] J. M. KAMINSKI. Synthesis and inhibition of human acrosin and trypsin and acute toxicity of aryl 4-guanidinobenzoates[J]. Journal of Medicinal Chemistry, 1986, 29 4: 514-519. DOI:10.1021/jm00154a015.
Properties of NPGB
| Melting point: | 241-243 °C |
| storage temp. | -20°C |
| form | Solid |
| color | White to off-white |
| BRN | 8024554 |
Safety information for NPGB
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for NPGB
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran
You may like
-
 4-Nitrophenyl 4-guanidinobenzoate hydrochloride CAS 19135-17-2View Details
4-Nitrophenyl 4-guanidinobenzoate hydrochloride CAS 19135-17-2View Details
19135-17-2 -
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5