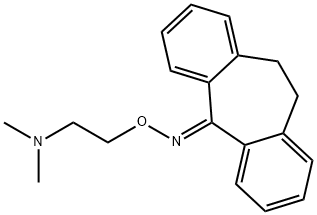
Noxiptiline
- CAS NO.:3362-45-6
- Empirical Formula: C19H22N2O
- Molecular Weight: 294.39
- MDL number: MFCD00865452
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:37
What is Noxiptiline?
Originator
Agedal,Bayer,W. Germany ,1969
Definition
ChEBI: Noxiptiline is an organic tricyclic compound.
Manufacturing Process
15 grams 5-keto-10,11-dihydrodibenzo-[a,d]cycloheptene dissolved in 225 ml of pyridine was mixed with 15 grams hydroxylamine hydrochloride, and the mixture was boiled under reflux for 22 hours. The bulk of the pyridine was then distilled off under reduced pressure, the residue was poured into water, and the aqueous mixture thus formed was extracted with ether.
The ether extract was washed with water, dried and heated to distill off the ether. The solid residue was recrystallized from a mixture of benzene and light petroleum (BP 40° to 60°C). 12.8 grams of the recrystallized oxime had a MP of 167° to 169°C.
A solution of 22 grams of the above described 5-oximino-10,11dihydrodibenzo-[a,d]cycloheptene in 120 ml benzene was treated with 7.8grams sodamide and the mixture was stirred and heated under reflux for 2 hours. At this stage, the 14.4 grams of hydrochloride of β-(dimethylamino) ethyl chloride was added and heating under reflux was continued for 16 hours. 50 ml water was then cautiously added to decompose unreacted sodamide and the benzene layer was separated and extracted with dilute (10%) aqueous hydrochloric acid.
The aqueous acid extracts were made alkaline with concentrated aqueous potassium hydroxide solution and then extracted with ether. The ether extracts were dried, the solvent was removed and the residual oil was distilled under reduced pressure. The product was 14.5 grams of the fraction boiling at 160° to 164°C, under a pressure of 0.05 mm of mercury.
Therapeutic Function
Psychostimulant
Properties of Noxiptiline
| Boiling point: | bp0.05 160-164° |
Safety information for Noxiptiline
Computed Descriptors for Noxiptiline
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran
![10,11-DIHYDRO-5 H-DIBENZO[A,D]CYCLOHEPTENE](https://img.chemicalbook.in/CAS/GIF/833-48-7.gif)
![10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-one oxime](https://img.chemicalbook.in/CAS/GIF/1785-74-6.gif)

You may like
-
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
 Cyclopentane carboxxylic acid 3400-45-1 99%View Details
Cyclopentane carboxxylic acid 3400-45-1 99%View Details
3400-45-1 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0