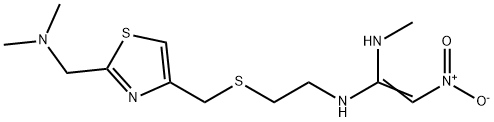
Nizatidine
Synonym(s):N-{2-[2-(N,N-Dimethylaminomethyl)-4-thiazolylmethylthio]ethyl}-N′-methyl-2-nitro-1,1-ethenediamine;Nizatidine
- CAS NO.:76963-41-2
- Empirical Formula: C12H21N5O2S2
- Molecular Weight: 331.46
- MDL number: MFCD00865660
- EINECS: 627-310-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-16 18:36:03
What is Nizatidine?
Absorption
Rapid (bioavailability of nizatidine exceeds 70%)
Toxicity
Oral, rat LD50: 301 mg/kg. Symptoms of overdose include cholinergic-type effects including lacrimation, salivation, emesis, miosis, and diarrhea.
Description
Nizatidine is the fifth H2-antagonist introduced to the world market as an antiulcer agent. It is reported to be effective in the treatment of both duodenal and gastric ulcers or the prevention of their recurrences. Given once-daily, nizatidine’s bioavailability is not diminished by the concurrent administration of an antacid.
Chemical properties
White Crystalline Powder
Originator
Lilly (USA)
The Uses of Nizatidine
Nizatidine is used for treating stomach and duodenum ulcers and other conditions accompanied by elevated acidity of the gastrointestinal tract.
The Uses of Nizatidine
Histamine H2-receptor antagonist related to Ranitidine (R120000). Antiulcerative.
The Uses of Nizatidine
immune suppressant, antineoplastic, antiviral
The Uses of Nizatidine
For the treatment of acid-reflux disorders (GERD), peptic ulcer disease, active benign gastric ulcer, and active duodenal ulcer.
Indications
For the treatment of acid-reflux disorders (GERD), peptic ulcer disease, active benign gastric ulcer, and active duodenal ulcer.
Background
A histamine H2 receptor antagonist with low toxicity that inhibits gastric acid secretion. The drug is used for the treatment of duodenal ulcers.
What are the applications of Application
Nizatidine is an inhibitor of gastric acid secretion
Definition
ChEBI: A member of the class of 1,3-thiazoles having a dimethylaminomethyl substituent at position 2 and an alkylthiomethyl moiety at position 4.
Indications
Nizatidine is the newest H2-receptor antagonist. Similar to ranitidine, it has a relative potency twice that of cimetidine.About 90% of an oral dose is absorbed, with a peak plasma concentration occurring after 0.5 to 3 hours; inhibition of gastric secretion is present for up to 10 hours.The elimination half-life is 1 to 2 hours, and more than 90% of an oral dose is excreted in the urine.
Manufacturing Process
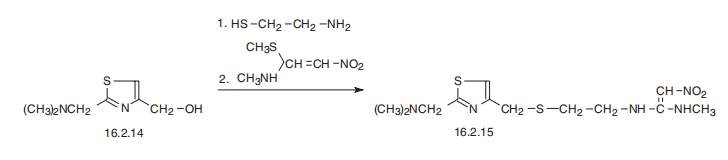
Nizatidine may be prepared by 2 ways.
1. A mixture of (25.7 g) 2-nitromethylenethiazolidine and acetonitrile (50 ml) was stirred and heated at 40°C under nitrogen. Methylamine gas (16.0 g) was passed into the stirred mixture over 45 minutes to give a solution. A slurry of 4-chloromethyl-2-dimethylaminomethylthiazole hydrochloride (40.0 g) (prepared as described in EP 49,618) in acetonitrile (50 ml) was added to the solution over a period of 4.5 hours whilst methylamine gas was bubbled through the reaction mixture such that methylamine (38.3 g) was added over the period (total methylamine added was 54.3 g). The temperature of the reaction mixture varied between 24° and 35°C during the addition. After the addition, the mixture was diluted with acetonitrile (50 ml) and stirred at ambient temperature for 17 hours. A solid was removed by filtration and the filtrate was split into 2 equal portions.
Portion 1: The solution was evaporated to give a black oil which was partitioned between water (200 ml) and chloroform (200 ml). The separated chloroform phase was washed with saturated brine, then dried over magnesium sulphate, filtered and evaporated to give a reddish oil which was dissolved in acetone (200 ml), boiled under reflux, cooled to 40°C and then seeded with nizatidine. The mixture was left to stand at 0°-5°C for 64 hours. The mixture was filtered to give nizatidine (10.4 g, 37%) m.p. 118-122°C. The structure was confirmed by1H NMR. The product was 95.4% pure by HPLC.
Portion 2: The mixture was evaporated to give an oil which was taken up in chloroform (200 ml) then washed with water (100 ml). The chloroform solution was washed with brine (100 ml), dried over magnesium sulphate, and then concentrated under reduced pressure at 45°C to give a brown oil. The oil was dissolved in acetone (200 ml) and activated charcoal (0.5 g) was added to the solution. The mixture was boiled under reflux for 10 minutes, then cooled to 45°C and filtered at this temperature to remove the charcoal. The filtrate was cooled to 20°C, seeded with nizatidine (0.05 g), then cooled 0°5°C for 45 minutes during which time crystallisation occurred. The mixture was filtered to give nizatidine (9.4 g, 32.2%).
2. A mixture of 2-nitromethylenethiazolidine (12.6 g) and water (30.0 ml) was stirred and heated at 40°C under argon. Methylamine (20.0 g of a 40% w/w aqueous solution) was added slowly over 30 minutes to the reaction mixture
at 40°C. The mixture was cooled at ambient temperature and further methylamine (23.6 g of 40% w/w aqueous solution) was added over 2.5 hours and a solution of 4-chloromethyl-2-dimethylaminomethylthiazole dihydrochloride (25.0 g) in water (30 ml) was added over 5.5 hours with the addition of the thiazole starting simultaneously with the addition of the methylamine. The reaction mixture was left to stir for a further 15 minutes and then was concentrated under reduced pressure. The solid obtained was dissolved in a mixture of methyl ethyl ketone (200 ml), aqueous potassium carbonate solution (43 ml, 10% w/w). The mixture was warmed slightly to obtain a solution. The mixture was separated and the aqueous layer was washed with methyl ethyl ketone (2 times 130 ml and then 1 times 100 ml). The combined organic layers were evaporated under reduced pressure to yield crude nizatidine (approximately 25.2 g), which was shown to be 89.4% pure by HPLC. The crude solid was dissolved in dichloromethane (300 ml). The solution was washed with water (3 times 75 ml). The combined aqueous layer and the washings were back extracted with dichloromethane and the combined organic layers were dried and concentrated under reduced pressure to give nizatidine (21.1 g, 74.3% yield). The solid was dissolved in ethanol (45 ml) by warming on a steam bath. The solution was removed from the steam bath treated with activated charcoal (2.3 g) and the mixture was boiled for a further 8 minutes. The mixture was hot filtered. The filtrate was cooled and filtered to give nizatidine (13.8 g, 48% yield) which was shown to be 99.8% pure by HPLC.
brand name
Axid (Braintree); Axid (Reliant).
Therapeutic Function
Antiulcer
General Description
Nizatidine, N-[2-[[[2-(dimethylamino)methyl]-4-thiazolyl]methyl]thio]-ethyl]-N'-methyl-2-nitro-1,1-ethenediamine (Axid), is an off-white to buff crystallinesolid that is soluble in water, alcohol, and chloroform. It isa thaizole derivative of raniditine and has pKas of 2.1 (side chain) and 6.8 (dimethylamino). Nizatidine’s mechanism ofaction is similar to other H2-antagonists, as is its receptor selectivity.It is more potent than cimetidine.
Nizatidine has excellent oral bioavailability (>90%).The effects of antacids or food on its bioavailability arenot clinically significant. The elimination half-life is 1 to2 hours. It is excreted primarily in the urine (90%) andmostly as unchanged drug (60%). Metabolites include nizatidinesulfoxide (6%), N-desmethylnizatidine (7%), andnizatidine N-oxide (dimethylaminomethyl function).Nizatidine has no demonstrable antiandrogenic action, effectson other hormones, or inhibitory effects on cytochromeisozymes involved in the metabolism of other drugs.
Pharmacokinetics
Nizatidine is a competitive, reversible inhibitor of histamine at the histamine H2-receptors, particularly those in the gastric parietal cells. By inhibiting the action of histamine on stomach cells, nizatidine reduces stomach acid production. Nizatidine had no demonstrable antiandrogenic action. Full-dose therapy for the problems treated by nizatidine lasts no longer than 8 weeks. It has been demonstrated that treatment with a reduced dose of nizatidine is effective as maintenance therapy following healing of active duodenal ulcers.
Clinical Use
H2 -receptor antagonist
Synthesis
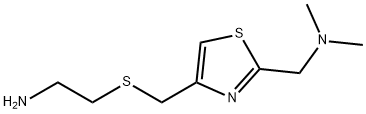
Nizatidine is N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl] thio] ethyl]-2-nitro-1,1-ethenediamine (16.2.15). According to its chemical structure, nizatidine is somewhat of a hybrid structure of ranitidine and famotidine, in which a side chain of ranitidine and carrying heterocycle, 2-aminothiazol, are used. Likewise, its synthesis also is a specific combination of pathways used for making both prototype drugs. 2-(Dimethylaminomethyl)- 4-hydroxymethylthiazol serves as the initial compound, from which the desired nizatidine (16.2.15) is synthesized by subsequent reaction with 2-mercaptoethylamine hydrochloride and then with N-methyl-1-methythio-2-nitroethenamine.
Veterinary Drugs and Treatments
While nizatidine acts similarly to cimetidine and ranitidine as an
H2 blocker to reduce gastric acid secretion in the stomach, in small
animal medicine its use has been primarily for its prokinetic effects.
It may be useful to treat delayed gastric emptying, pseudo-obstruction
of the intestine and constipation.
H2 blockers may be useful in preventing hemorrhagic necrosis
in feline pancreatitis.
Drug interactions
Potentially hazardous interactions with other drugs
Antifungals: absorption of itraconazole, ketoconazole
and possibly posaconazole reduced, avoid with
posaconazole suspension.
Antivirals: concentration of atazanavir reduced;
concentration of raltegravir possibly increased -
avoid; avoid for 12 hours before and 4 hours after
rilpivirine.
Cytotoxics: avoid with dasatinib and erlotinib;
possibly reduced absorption of pazopanib - give at
least 2 hours before or 10 hours after nizatidine;
possibly reduced absorption of lapatinib.
Ulipristal: contraceptive effect possibly reduced -
avoid with high dose ulipristal.
Metabolism
Hepatic. Less than 7% of an oral dose is metabolized as N2-monodes-methylnizatidine, an H2-receptor antagonist, which is the principal metabolite excreted in the urine. Other likely metabolites are the N2-oxide (less than 5% of the dose) and the S-oxide (less than 6% of the dose).
Metabolism
A small amount of nizatidine is metabolised in the liver: nizatidine N-2-oxide, nizatidine S-oxide, and N-2- monodesmethylnizatidine have been identified, the latter having about 60% of the activity of nizatidine. More than 90% of a dose of nizatidine is excreted in the urine, in part by active tubular secretion, within 12 hours, about 60% as unchanged drug. Less than 6% is excreted in the faeces
Properties of Nizatidine
| Melting point: | 130-1320C |
| Boiling point: | 478.2±45.0 °C(Predicted) |
| Density | 1.249±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Sparingly soluble in water, soluble in methanol. |
| form | neat |
| pka | 2.1, 6.8(at 25℃) |
| form | Solid |
| color | Off-White to Pale Yellow |
| Water Solubility | 21.4g/L(temperature not stated) |
| Merck | 14,6660 |
| CAS DataBase Reference | 76963-41-2(CAS DataBase Reference) |
Safety information for Nizatidine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Nizatidine
Nizatidine manufacturer
Solara Active Pharma Sciences Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![N'-[2-[[[2-[(DiMethylaMino)Methyl]-4-thiazolyl]Methyl]thio]ethyl] Nizatidine](https://img.chemicalbook.in/CAS/GIF/1193434-63-7.gif)
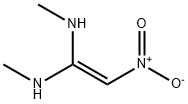
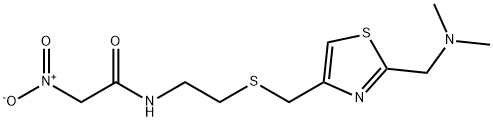
![N1,N1'-[2,4-Thiazolediylbis(Methylenethio-2,1-ethanediyl)]bis(N'-Methyl-2-nitro-1,1-ethenediaMine)](https://img.chemicalbook.in/CAS/GIF/1193434-62-6.gif)

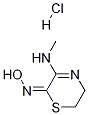
You may like
-
 76963-41-2 Nizatidine 98%View Details
76963-41-2 Nizatidine 98%View Details
76963-41-2 -
 76963-41-2 98%View Details
76963-41-2 98%View Details
76963-41-2 -
 Nizatidine 95% CAS 76963-41-2View Details
Nizatidine 95% CAS 76963-41-2View Details
76963-41-2 -
 Nizatidine CAS 76963-41-2View Details
Nizatidine CAS 76963-41-2View Details
76963-41-2 -
 Nizatidine CAS 76963-41-2View Details
Nizatidine CAS 76963-41-2View Details
76963-41-2 -
 Nizatidine 76963-41-2 98%View Details
Nizatidine 76963-41-2 98%View Details
76963-41-2 -
 Nizatidine CAS 76963-41-2View Details
Nizatidine CAS 76963-41-2View Details
76963-41-2 -
 Nizatidine CAS 76963-41-2View Details
Nizatidine CAS 76963-41-2View Details
76963-41-2