Nitrofurazone
Synonym(s):Nitrofurazone;Nitrofural;5-Nitro-2-furaldehyde semicarbazone;NZ;Furacin
- CAS NO.:59-87-0
- Empirical Formula: C6H6N4O4
- Molecular Weight: 198.14
- MDL number: MFCD00003225
- EINECS: 200-443-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:18
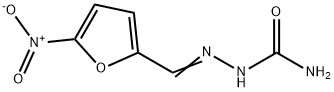
What is Nitrofurazone?
Absorption
Well absorbed.
Toxicity
Rat LD50 = 590 mg/kg; Allergic contact dermatitis is the most frequently reported adverse effect, occurring in approximately 1 % of patients treated.
Description
Nitrofurazone is an antibacterial agent used in animal feed. Occupational dermatitis was reported in cattle breeders and farmers.
Chemical properties
white to light yellow crystal powde
Originator
Furacin,Norwich Eaton ,US,1946
The Uses of Nitrofurazone
Nitrofurazone is a topical anti-infective and bactericide for most pathogens commonly causing surface infections; in the adjunctive therapy of patients with second and third degree bums when bacterial resistance to other agents is areal or potential problem; in skin grafting where bacterial contamination may cause graft rejection and/or donor-site infection; antibacterial agent for the treatment or prevention of infections in a variety of conditions involving skin, eyes, ears, nose and genito-urinary tract; used on pyodermas, ulcers, and wounds; affects some protozoa and is an effective prophylaxis against nosocomial infections; antiseptic lubricant for trans urethral resection; anti-microbial in veterinary medicine.
The Uses of Nitrofurazone
Anti-infective (topical). Antimicrobial.Environmental contaminants; Food contaminants; Heat processing contaminants
Background
Nitrofural or nitrofurazone is a topical anti-infective agent effective against gram-negative and gram-positive bacteria. It is used for superficial wounds, burns, ulcers, and skin infections. Nitrofural has also been administered orally in the treatment of trypanosomiasis.
Except for topical drug products formulated for dermatologic application, the FDA withdrew its approval for the use of drug products containing nitrofurazone.
Indications
For the treatment of bacterial skin infections including pyodermas, infected dermatoses and infections of cuts, wounds, burns and ulcers due to susceptible organisms.
Definition
ChEBI: A semicarbazone resulting from the formal condensation of semicarbazide with 5-nitrofuraldehyde. A broad spectrum antibacterial drug, although with little activity against Pseudomonas species, it is used as a local application for burns, ulcer , wounds and skin infections.
Definition
A type of organic compound containing the C:N.NH.CO.NH2 grouping, formed by reaction of an aldehyde or ketone with semicarbazide (H2N.NH.CO.NH2). The compounds are crystalline solids with sharp melting points, which can be used to characterize the original aldehyde or ketone.
Indications
Nitrofurazone (Furacin), a synthetic nitrofuran derivative with a broad antibacterial spectrum. Although its exact mechanism of action is unknown, it is thought to inhibit bacterial enzymes involved in carbohydrate metabolism. It is not effective against fungal or viral organisms. It is used as adjunctive therapy in patients with second- and third-degree burns when bacterial resistance to other antiinfective agents is a potential problem. It is not effective in the treatment ofminor burns or superficial bacterial infections involving wounds, cutaneous ulcers, or various pyodermas. It is rarely used by dermatologists as it carries a high risk of acquired contact sensitivity.
Manufacturing Process
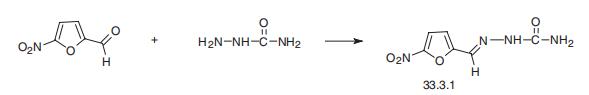
A mixture of 43 grams of semicarbazide hydrochloride and 31 grams of sodium acetate is dissolved in 150 cc of water. The pH of this solution is approximately 5. Ethyl alcohol (95% by volume) in the amount of 250 cc is added and the mixture is stirred mechanically. A solution of 53.5 grams of carefully purified 2-formyl-5-nitrofuran in 250 cc of the said alcohol is added dropwise to the semicarbazide solution at room temperature. After completing the addition of the aldehyde solution, the mixture is stirred for another hour. The precipitate is removed from the reaction mixture by filtration. It is washed well with ethyl alcohol and dried to constant weight at 70°C in an oven. The product weighs 73 grams, corresponding to a yield of 97%. It is obtained in the form of pale yellow needles, which are not subjected to further purification, according to US Patent 2,416,234.
brand name
Actin-N (Sherwood); Furacin (Shire);Acmor-s;Akutol;Anginofur;Auroid;Beca furazona;Bifuran;Burnazone;Dermobion;Ectofural;Escofuran;Escofuron;Fluorobioptal;Furacilinum;Furacinas;Furacinethin;Furacin-sol;Furacin-streusol;Furacocid;Furacol;Furaseptin;Fura-septin;Fura-vet;Furea;Furesan;Furotalgin;Furovol;Germax;Germex;Ginejuvent;I fomula;Ii formula;Kamfomen;Kindrog;Lifuzol;Mammiject;Mastidol;Muldacin;Neovagon;Nfz 1;Nitocetin;Nitrocol plus;Nitro-rea;Notaba;Sanifur;Scandantin;Sulfamyton-n;Taristop;Tranoxa;Tuocurine;Urafadyn;Uroletten;Viropulver;Yalrocin;Zoppin spray blu.
Therapeutic Function
Topical antiinfective
World Health Organization (WHO)
Nitrofural, a nitrofuran derivative with broad-spectrum antibacterial activity, was introduced in the early 1940s for the topical treatment of various skin conditions. It has also been used systemically for the treatment of African trypasonomiasis. Following recent findings of in vitro mutagenicity and of carcinogenicity in experimental animals, use of topical preparations containing this substance was restricted in Germany. Nitrofural remains registered in several countries and the World Health Organization is not aware of restrictive action having been taken elsewhere.
General Description
Odorless pale yellow needles or yellow powder. pH (saturated aqueous solution) 6.0 - 6.5. Alkaline solutions are dark orange.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Furacilin darkens on prolonged exposure to light. Furacilin can react violently with reducing materials. .
Fire Hazard
Flash point data for Furacilin are not available; however, Furacilin is probably combustible.
Pharmaceutical Applications
A synthetic compound used in the topical treatment of wounds and burns and as an instillation for bladder washout. A nitrofurazone-impregnated urinary catheter is said to reduce infection in catheterized patients. Activity against the common bacterial pathogens is sufficient to cover most pathogens that cause infections of burns and wounds, with the important exception of Ps. aeruginosa. Attention has been drawn to its activity against methicillin-resistant Staphylococcus aureus, and its use in clearing carriage has been suggested. Slight absorption occurs from intact skin (c. 1%) and burned skin (5%). It is neither a primary irritant nor a sensitizer, but some preparations contain polyethylene glycol as a vehicle, and absorption can cause problems in patients with reduced renal function. Of limited availability.
Contact allergens
Nitrofurazone is an antibacterial agent used in animal feeds. Occupational dermatitis was reported in cattle breeders or farmers.
Pharmacokinetics
Nitrofurazone is a topical antibacterial agent indicated as an adjunctive therapy for second and third degree burns when resistance to other agents is a real or potential problem. Nitrofurazone is also indicated in skin grafting when bacterial contamination may cause graft rejection or donor site infection, especially in hospitals with a history of resistant bacteria.
Clinical Use
5-Nitro-2-furaldehyde semicarbazone (Furacin) occurs asa lemon-yellow crystalline solid that is sparingly solublein water and practically insoluble in organic solvents.Nitrofurazone is chemically stable, but moderately lightsensitive.It is used topically in the treatment of burns, especiallywhen bacterial resistance to other agents may be a concern.It may also be used to prevent bacterial infection associatedwith skin grafts. Nitrofurazone has a broad spectrumof activity against Gram-positive and Gram-negative bacteria,but it is not active against fungi. It is bactericidalagainst most bacteria commonly causing surface infections,including S. aureus, Streptococcus spp., E. coli,Clostridium perfringens, Enterobacter (Aerobacter) aerogenes,and Proteus spp.; however, P. aeruginosa strainsare resistant.Nitrofurazone is marketed in solutions, ointments, andsuppositories in a usual concentration of 0.2%.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by subcutaneous route. Questionable carcinogen with experimental carcinogenic, neoplas tigenic, tumorigenic, and teratogenic data, Experimental reproductive effects. A human sensitizer. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Nitrofurazone is the semicarbazone 5-nitrofurfurol (33.3.1). It is synthesized by reacting 5-nitrofurfurol with semicarbazide.
Veterinary Drugs and Treatments
Nitrofurazone can be used topically as an antibacterial for treating or preventing superficial infections. It is a nitrofuran antibacterial that is bactericidal for many bacteria, including E. Coli, Staph aureus, etc. Nitrofurazone’s mechanism of action is thought to be associated with inhibiting bacterial enzymes that primarily degrade glucose and pyruvate.
Metabolism
Nitrofurans, including nitrofural, undergo metabolic reduction at the nitro group to generate reactive species which can covalently bind to cellular macromolecules (Polnaszek et al., 1984; Kutcher & McCalla, 1984; McCalla 1979; McCalla et al., 1975).
Properties of Nitrofurazone
| Melting point: | 242-244 °C (lit.) |
| Boiling point: | 335.43°C (rough estimate) |
| Density | 1.6031 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| Flash point: | 2 °C |
| storage temp. | 2-8°C |
| solubility | Very slightly soluble in water, slightly soluble in ethanol (96 per cent). |
| form | neat |
| pka | pKa 9.28± 0.03( EtOH,t =35±0.1,I=0.00) (Uncertain) |
| form | Solid |
| color | Yellow to Dark Yellow |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| Sensitive | Light Sensitive |
| Merck | 14,6600 |
| BRN | 86403 |
| CAS DataBase Reference | 59-87-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 5-Nitro furfural semicarbazone(59-87-0) |
| IARC | 3 (Vol. 50) 1990 |
| EPA Substance Registry System | Nitrofurazone (59-87-0) |
Safety information for Nitrofurazone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H341:Germ cell mutagenicity H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Nitrofurazone
| InChIKey | IAIWVQXQOWNYOU-FPYGCLRLSA-N |
Nitrofurazone manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 NITROFURAZONE 99%View Details
NITROFURAZONE 99%View Details -
 Nitrofurazone CAS 59-87-0View Details
Nitrofurazone CAS 59-87-0View Details
59-87-0 -
 Nitrofurazone CAS 59-87-0View Details
Nitrofurazone CAS 59-87-0View Details
59-87-0 -
 Nitrofurazone CAS 59-87-0View Details
Nitrofurazone CAS 59-87-0View Details
59-87-0 -
 Nitrofural CAS 59-87-0View Details
Nitrofural CAS 59-87-0View Details
59-87-0 -
 5-Nitro-2-furaldehyde semicarbazone CAS 59-87-0View Details
5-Nitro-2-furaldehyde semicarbazone CAS 59-87-0View Details
59-87-0 -
 Nitrofurazone API PowderView Details
Nitrofurazone API PowderView Details
59-87-0 -
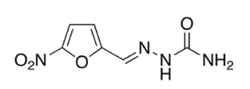 99% Nitrofurazone, Analytical GradeView Details
99% Nitrofurazone, Analytical GradeView Details
59-87-0
