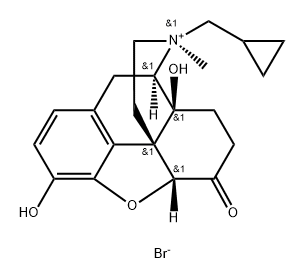
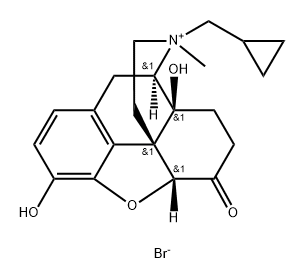
Naltrexone methylbromide
Synonym(s):MNTX;Naltrexone MB;N-Methylnaltrexone;Quaternary ammonium naltrexone;17-(Cyclopropylmethyl)-4,5α-epoxy-3,14-dihydroxy-17-methyl-6-oxomorphinanium bromide
- CAS NO.:73232-52-7
- Empirical Formula: C21H26BrNO4
- Molecular Weight: 436.35
- MDL number: MFCD06407831
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Naltrexone methylbromide?
Description
The widespread efficacy of opioids in treating patients with moderate to
severe acute and chronic pain is often accompanied by untoward side
effects. In particular, opioid-induced bowel dysfunction is one of the
more common and debilitating consequences afflicting up to 50% of
patients. To counteract the peripherally-mediated adverse effects, opioid
antagonists such as naloxone, naltrexone, and nalmephene are sometimes prescribed. The latest market entry exploits a strategic
modification of naltrexone to lower its lipid solubility and increase its
polarity: quaternization of the amine of naltrexone by methylation
(methyl bromide) prevents crossing of the blood–brain barrier thereby
creating an effective peripheral antagonist. Despite a loss of potency
upon methylation, methylnaltrexone antagonizes opioid binding at
m-opioid receptors with an IC50 of 70 nM. Its affinity for k-opioid receptors
is approximately eightfold less (IC50= 575 nM) with no significant binding
to d-opioid, orphanin, or non-opioid receptors. Methylnaltrexone bromide
has been approved for the treatment of opioid-induced constipation in
patients with advanced illness receiving palliative care.Regarding metabolism, methylnaltrexone bromide is eliminated primarily as intact drug (85% based on administered radioactivity) by slightly more renal than hepatic clearance.
The most common adverse events were abdominal pain and flatulence followed by nausea, dizziness, and diarrhea.
Chemical properties
Pale Pink Solid
Originator
University of Chicago (United States)
The Uses of Naltrexone methylbromide
A metabolite of Naltrexone (N285750). Methylnaltrexone (MNTX), a selective μ-opioid receptor antagonist, functions as a peripherally acting receptor antagonist in tissues of the gastrointestinal tract.
The Uses of Naltrexone methylbromide
Methylnaltrexone bromide has been used as a drug to measure plasma protein binding (PPB), permeability (Pm) and the membrane coefficient (KIAM) for the prediction of blood brain barrier (BBB) penetration. It is also used as a mu-opioid receptor (MOR) antagonist to abrogate morphine tolerance and opioid-induced hyperalgesia (OIH).
brand name
Relistor
General Description
Methylnaltrexone does not cross blood brain barrier and does not affect the opioid effects in the brain, such as analgesia. It is used to treat opioid-induced constipation (OIC).
Biochem/physiol Actions
Methylnaltrexone bromide is a narcotic antagonist. It is a peripheral mu-opiod receptor antagonist that cannot cross the blood-brain barrier. It reverses many opioid side-effects without interfering with pain relief.
Clinical Use
N-Methylnaltrexone is the polar derivative of naltrexone, which does not reach the central nervous system but can block intestinal opioid receptors. The compound is under development to counteract opioid induced bowel dysfunction such as constipation and megacolon .
Synthesis
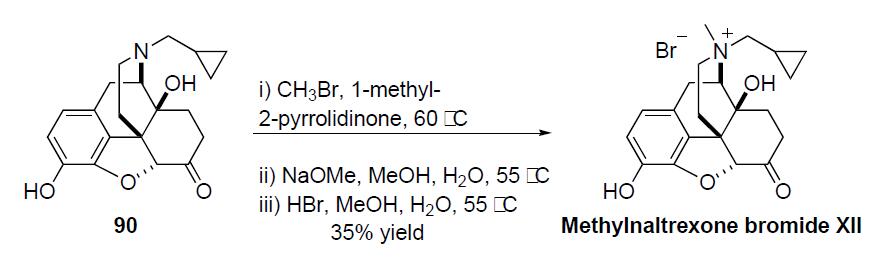
The synthesis of methylnaltrexone bromide proceeds in a straightforward manner via the alkylation of naltrexone 90 in the following scheme. Naltrexone 90 was reacted with methyl bromide in 1-methyl-2-pyrrolidinone at 60 ??C. The resulting crude product was treated with sodium methoxide in methanol/ water at 55 ??C to remove any undesired phenolic (Oalkylated) side-products. The resulting crude sodium salt was treated with hydrobromic acid in methanol/water and upon crystallization gave methylnaltrexone bromide (XII) in 35% overall yield.
storage
Store at -20°C
Properties of Naltrexone methylbromide
| Melting point: | 237-239°C |
| storage temp. | -20°C |
| solubility | H2O: ≥5mg/mL |
| form | powder |
| color | white to beige |
| Stability: | Hygroscopic |
Safety information for Naltrexone methylbromide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Naltrexone methylbromide
Naltrexone methylbromide manufacturer
Rusan Pharma Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 73232-52-7 Methylnaltrexone bromide 98%View Details
73232-52-7 Methylnaltrexone bromide 98%View Details
73232-52-7 -
 Methylnaltrexone bromide CAS 73232-52-7View Details
Methylnaltrexone bromide CAS 73232-52-7View Details
73232-52-7 -
 Methylnaltrexone bromide CAS 73232-52-7View Details
Methylnaltrexone bromide CAS 73232-52-7View Details
73232-52-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1