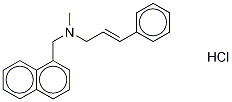
Naftifine hydrochloride
- CAS NO.:65473-14-5
- Empirical Formula: C21H22ClN
- Molecular Weight: 323.86
- MDL number: MFCD00059047
- EINECS: 620-502-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
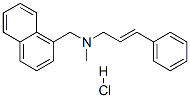
What is Naftifine hydrochloride?
Description
Naftifine hydrochloride is a topical antifungal agent which acts via inhibiting squalene epoxidase. It demonstrates good activity against Trichophyton, Epidermophyton, Microsporum, Aspergillus, and species. It is the prototype of a significantly improved series of antifungal agents, exemplified by SF 86-327 in which the phenyl group of naftifine has been replaced by t-butylace tylene.
Chemical properties
White Powder
Originator
Sandoz (Switzerland)
The Uses of Naftifine hydrochloride
An antifungal agent
The Uses of Naftifine hydrochloride
Antimycotic allylamine. An antifungal (topical) agent.
What are the applications of Application
Naftifine, Hydrochloride is an antifungal agent
Definition
ChEBI: Naftifine hydrochloride is a hydrochloride and an allylamine antifungal drug.
brand name
Naftin (Merz);EXODERIL.
General Description
N-Methyl-N-(3-phenyl2-propenyl)-1-naphthalenemethanaminehydrochloride (Naftin) is a white crystallinepowder that is soluble in polar solvents such asethanol and methylene chloride. It is supplied in a 1% concentrationin a cream and in a gel for the topical treatmentof ringworm, athlete’s foot, and jock itch. Although unapprovedfor these uses, naftifine has shown efficacy fortreatment of ringworm of the beard, ringworm of the scalp,and tinea versicolor.
Clinical Use
Naftifine hydrochloride (Naftin) is available for topical use only in the treatment of cutaneous dermatophyte and Candida infections; it is as effective as topical azoles for these conditions.
Synthesis
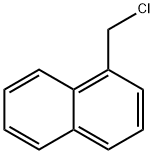
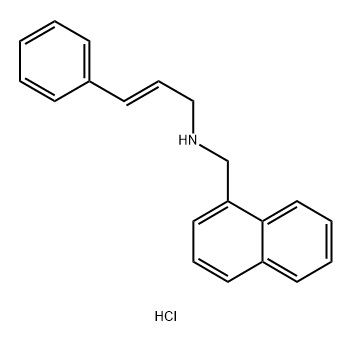
Naftifine hydrochloride is prepared by the reaction of (E)-N-methylcinnamylamine and 1-Chloromethylnaphthalene. The steps are as follows:
Step 1: (E)-N-methylcinnamylamine; 1-Chloromethylnaphthalene With sodium hydroxide In toluene at 86℃; for 6h;
Step 2: With hydrogenchloride In water; toluene at 15 - 20℃; for 3.5h;
Step 3: Add toluene (75 mL) to the yellow oil of step 2 (trans-N-cinnamomethylamine crude)And 15% NaOH (9.8g),Warming up,Stir well,When the temperature rises to 86 ° C,1-Chloromethylnaphthalene (11.8 g,100.2mmol), dripping,The reaction was incubated at 86 ° C for 6 h.Medium controlled N-cinnamylmethylamine is less than 0.5%,Cool down to 30 ° C, add water (75mL) to the system,Stirring and standing, separating the organic phase,The organic phase was washed with water (75 mL).Allow to stand and separate the organic phase, Concentrated hydrochloric acid (7.0 g) was added to the organic phase in a 15 ° C ice water bath.Adjust the pH to about 2 and stir for 0.5 h.After stirring at 20 ° C for 3 h, the filter cake was filtered.The filter cake was rinsed with toluene (20 mL).Obtained a wet cake (reduced naftifine hydrochloride) 19.5g;The wet crude product obtained in step 3 (crude naftifine hydrochloride) was added to isopropyl alcohol (38.6 g).Warmed to 85 ° C, dissolved,Slowly cool after dissolving,The cooling rate is 10 °C / h,A small amount of solid precipitated at 35 ° C.Crystallization at this temperature for 2 h,Continue to cool down to 20 ° C,Insulation for 3h,filter,Get the wet cake,The filter cake was dried under vacuum at 45 ° C.Obtained 16.2g of finished naftifine hydrochloride,The purity of the finished naftifine hydrochloride was 99.4% (HPLC normalization method).The product yield was 68.0%.The product yield is the molar yield,The calculation is as follows:Product yield (%)={m/[(m0/M0)*M]}*100%Symbol Description: m is the mass of the finished product, g; m0 is the mass of cinnamyl alcohol, g; M is the molar mass of naftifine hydrochloride,g/mol; M0 is the molar mass of cinnamyl alcohol, g/mol.
Properties of Naftifine hydrochloride
| Melting point: | 172-175°C |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | ethanol: soluble50mg/mL |
| form | Solid |
| color | White to Off-White |
| Merck | 14,6355 |
| InChI | InChI=1S/C21H21N.ClH/c1-22(16-8-11-18-9-3-2-4-10-18)17-20-14-7-13-19-12-5-6-15-21(19)20;/h2-15H,16-17H2,1H3;1H/b11-8+; |
| CAS DataBase Reference | 65473-14-5(CAS DataBase Reference) |
Safety information for Naftifine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Naftifine hydrochloride
| InChIKey | OLUNPKFOFGZHRT-YGCVIUNWSA-N |
| SMILES | N(C/C=C/C1=CC=CC=C1)(C)CC1=C2C(C=CC=C2)=CC=C1.[H]Cl |
Abamectin manufacturer
Synergene Active Ingredients Pvt. Ltd.
Acute Research
Ralington Pharma
Allastir Private Limited
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
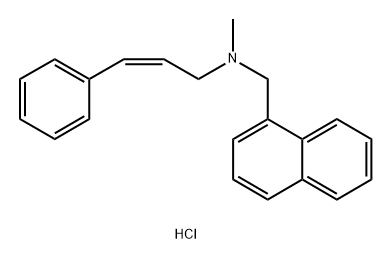
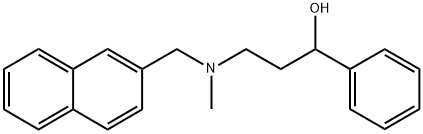
![α-[2-[Methyl(1-naphthalenylmethyl)amino]ethyl]-Benzenemethanol](https://img.chemicalbook.in/)
You may like
-
 65473-14-5 99%View Details
65473-14-5 99%View Details
65473-14-5 -
 Naftifine hydrochloride 98%View Details
Naftifine hydrochloride 98%View Details
65473-14-5 -
 Naftifine HCl 95.00% CAS 65473-14-5View Details
Naftifine HCl 95.00% CAS 65473-14-5View Details
65473-14-5 -
 Naftifine HCl >98% (HPLC) CAS 65473-14-5View Details
Naftifine HCl >98% (HPLC) CAS 65473-14-5View Details
65473-14-5 -
 Naftifine Hydrochloride CAS 65473-14-5View Details
Naftifine Hydrochloride CAS 65473-14-5View Details
65473-14-5 -
 Naftifine hydrochloride 65473-14-5 98%View Details
Naftifine hydrochloride 65473-14-5 98%View Details
65473-14-5 -
 Naftifine hydrochloride CAS 65473-14-5View Details
Naftifine hydrochloride CAS 65473-14-5View Details
65473-14-5 -
 Naftifine hydrochloride CAS 65473-14-5View Details
Naftifine hydrochloride CAS 65473-14-5View Details
65473-14-5