N-Hydroxyphthalimide
- CAS NO.:524-38-9
- Empirical Formula: C8H5NO3
- Molecular Weight: 163.13
- MDL number: MFCD00005891
- EINECS: 208-358-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-20 18:06:30
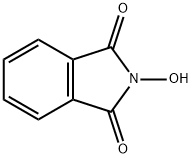
What is N-Hydroxyphthalimide?
Chemical properties
N-Hydroxyphthalimide is yellow moist powder
The Uses of N-Hydroxyphthalimide
Aerobic oxidation of various alcohols has been accomplished by using a new catalytic system, N-hydroxyphthalimide (NHPI) combined with Co (acac). A practical catalytic method to convert alkylbenzenes into the corresponding carboxylic acids under atmospheric dioxygen at ambient temperature using a combined catalytic system consisting of N-hydroxyphthalimide (NHPI) and Co (OAc) 2 was developed. Novel catalysis by N-hydroxyphthalimide in the oxidation of organic substrates by molecular oxygen was described. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Purely organic and catalytic systems of anthraquinones and N-hydroxyphthalimide efficiently promote oxygenation of hydrocarbons with dioxygen under mild conditions. Hydroxylation of polycyclic alkanes with molecular oxygen catalyzed by N-hydroxyphthalimide (NHPI) combined with was transition metal salts.
The Uses of N-Hydroxyphthalimide
N-Hydroxyphthalimide (NHPI) is a versatile biochemical with applications that extend beyond its role as a catalyst in oxidation reactions. It is notably used as an additive in the DCC method of peptide synthesis, where it enhances the yield of optically pure products. In research, NHPI is recognized for its utility as a biochemical, contributing to the advancement of scientific studies. Moreover, it plays a significant role in the pharmaceutical industry, being instrumental in the synthesis of a new class of antibacterials that are potent against macrolide-resistant bacteria. Additionally, NHPI is utilized in the synthesis of various heterocyclic compounds, including pyrazolidines, isoxazolidines, and tetrahydrooxazines, showcasing its broad spectrum of applications in the field of organic chemistry and medicinal chemistry.
Synthesis Reference(s)
Tetrahedron Letters, 37, p. 2035, 1996 DOI: 10.1016/0040-4039(96)00211-0
Description
N-hydroxyphthalimide (NHPI) is an organocatalyst which is attracting a growing interest in the field of C-H bond activation. Initially reported by Grochowsky in 1977 and subsequently studied by Masui, NHPI proved effective in mediating a wide range of free-radical transformations via hydrogen atom transfer (HAT) processes. Despite a few works reporting its use for forming carbon-carbon, carbon-nitrogen, and carbon-silicon bonds, NHPI has found its main application in promoting aerobic oxidation reactions. A fundamental contribution to this field was made by Ishii and co-workers, who first reported using NHPI to promote the aerobic oxidation of alcohols and hydrocarbons to the corresponding carbonylic and/or carboxylic derivatives.
Purification Methods
N -Hydroxyphthalimide [524-38-9] M 163.1, m 230o, ~235o(dec), 237-240o, 7.0. Dissolve the imide in H2O by adding Et3N to form the salt and while hot, acidify, cool and pour into a large volume of H2O. Filter off the solid, wash it with H2O and dry it over P2O5 in a vacuum. [Nefken & Teser J Am Chem Soc 83 1263 1961, Fieser 1 485 1976, Nefkens et al. Recl Trav Chim, Pays-Bas 81 683 1962] The O-acetyl derivative has m 178-180o (from EtOH). [Beilstein 21/11 V 100.]
Properties of N-Hydroxyphthalimide
| Melting point: | 233 °C (dec.) (lit.) |
| Boiling point: | 290.19°C (rough estimate) |
| Density | 1.64 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5510 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 6.10±0.20(Predicted) |
| form | Powder |
| color | Yellow |
| Water Solubility | Slightly soluble in water. |
| Merck | 14,4838 |
| BRN | 131208 |
| CAS DataBase Reference | 524-38-9(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Isoindole-1,3(2H)-dione, 2-hydroxy- (524-38-9) |
Safety information for N-Hydroxyphthalimide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for N-Hydroxyphthalimide
| InChIKey | CFMZSMGAMPBRBE-UHFFFAOYSA-N |
N-Hydroxyphthalimide manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 524-38-9 N-Hydroxy phthalimide 98%View Details
524-38-9 N-Hydroxy phthalimide 98%View Details
524-38-9 -
 524 - 38 - 9 99%View Details
524 - 38 - 9 99%View Details
524 - 38 - 9 -
 N-Hydroxyphthalimide CAS 524-38-9View Details
N-Hydroxyphthalimide CAS 524-38-9View Details
524-38-9 -
 N-Hydroxyphthalimide 98% CAS 524-38-9View Details
N-Hydroxyphthalimide 98% CAS 524-38-9View Details
524-38-9 -
 N-Hydroxyphthalimide pure CAS 524-38-9View Details
N-Hydroxyphthalimide pure CAS 524-38-9View Details
524-38-9 -
 N-Hydroxyphthalimide, 97% CAS 524-38-9View Details
N-Hydroxyphthalimide, 97% CAS 524-38-9View Details
524-38-9 -
 N-Hydroxy Phthalimide CAS 524-38-9View Details
N-Hydroxy Phthalimide CAS 524-38-9View Details
524-38-9 -
 Chemical Grade N - Hydroxy Phthalimide, Packaging Type: 1 KgView Details
Chemical Grade N - Hydroxy Phthalimide, Packaging Type: 1 KgView Details
524-38-9
