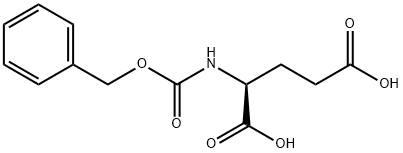
N-Benzyloxycarbonyl-L-proline
Synonym(s):Z-L -Proline
- CAS NO.:1148-11-4
- Empirical Formula: C13H15NO4
- Molecular Weight: 249.26
- MDL number: MFCD00003170
- EINECS: 214-557-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
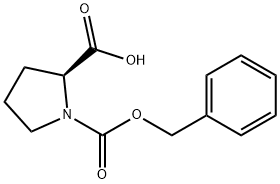
What is N-Benzyloxycarbonyl-L-proline?
Chemical properties
white to light yellow crystal powder
The Uses of N-Benzyloxycarbonyl-L-proline
N-(Benzyloxycarbonyl)-L-proline is a potent in vitro and in vivo inhibitor of prolidase; a specific peptidase that cleaves dipeptides with a C-terminal prolyl and hydroxylprolyl residue. It is also used in the synthesis of N-(L-Prolyl)-β-alanine which is a derivative of the naturally occurring beta amino acid β-Alanine.
Preparation
Caution! All procedures must be carried out in an efficient fume cupboard, wearing latex gloves and chemical-proof safety goggles. ι-Proline (10.0 g, 8.7 mmol) was dissolved in 2 m sodium hydroxide solution (40 mL), and the solution was cooled to ice-water temperature. Z-Cl (20.5 g, 12 mmol) was added portionwise, with vigorous stirring or occasional shaking, over a period of 30 min at 0–5 °C to the solution of l-proline. The ice bath was then removed and stirring or occasional shaking was continued for 30 min while the reaction mixture warmed to room temperature. The mixture was then acidified to Congo red by the gradual addition of concentrated hydrochloric acid, and the oil was extracted into ethyl acetate. The extract was dried over magnesium sulfate, the mixture was filtered, and the filtrate was concentrated in vacuo. The residue was extracted/triturated with warm tetrachloromethane. The washings were decanted and the residue was further purified by recrystallization from ethyl acetate/petroleum ether.
Effects
Results demonstrated that N-benzyloxycarbonyl-l-proline (Cbz-Pro) is a potent inhibitor of prolidase in cultured fibroblasts and it can be used in vivo to better characterize the prolidase enzyme and further investigate PD physiopathology.
Properties of N-Benzyloxycarbonyl-L-proline
| Melting point: | 75-77 °C |
| Boiling point: | 392.36°C (rough estimate) |
| alpha | -60 º (c=2,AcOH) |
| Density | 1.1952 (rough estimate) |
| refractive index | -40 ° (C=2, EtOH) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Solubility in methanol, almost transparency. |
| form | Crystalline Powder or Crystals |
| pka | 3.99±0.20(Predicted) |
| color | White to off-white |
| optical activity | [α]20/D 42°, c = 2 in ethanol |
| BRN | 88579 |
| CAS DataBase Reference | 1148-11-4(CAS DataBase Reference) |
Safety information for N-Benzyloxycarbonyl-L-proline
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for N-Benzyloxycarbonyl-L-proline
| InChIKey | JXGVXCZADZNAMJ-NSHDSACASA-N |
N-Benzyloxycarbonyl-L-proline manufacturer
JSK Chemicals
Astrix Pharmaceuticals
ASM Organics
Related products of tetrahydrofuran
You may like
-
![1148-11-4 N-Cbz-L-proline, 99% [Z-Pro-OH] 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/ac3f52a0-a78a-4c4a-bb43-0fc3e8e504f1.png) 1148-11-4 N-Cbz-L-proline, 99% [Z-Pro-OH] 98%View Details
1148-11-4 N-Cbz-L-proline, 99% [Z-Pro-OH] 98%View Details
1148-11-4 -
 N-Benzyloxycarbonyl-L-proline 1148-11-4 98%View Details
N-Benzyloxycarbonyl-L-proline 1148-11-4 98%View Details
1148-11-4 -
 Z-Pro-OH 98% CAS 1148-11-4View Details
Z-Pro-OH 98% CAS 1148-11-4View Details
1148-11-4 -
 N-Benzyloxycarbonyl-L-proline CAS 1148-11-4View Details
N-Benzyloxycarbonyl-L-proline CAS 1148-11-4View Details
1148-11-4 -
 1148-11-4 ((benzyloxy)carbonyl)-L-proline 98.0%View Details
1148-11-4 ((benzyloxy)carbonyl)-L-proline 98.0%View Details
1148-11-4 -
 N-Benzyloxycarbonyl-L-proline CAS 1148-11-4View Details
N-Benzyloxycarbonyl-L-proline CAS 1148-11-4View Details
1148-11-4 -
 Z-L-Proline CAS 1148-11-4View Details
Z-L-Proline CAS 1148-11-4View Details
1148-11-4 -
 Z-Pro-OH CAS 1148-11-4View Details
Z-Pro-OH CAS 1148-11-4View Details
1148-11-4