N-AMYL NITRATE
- CAS NO.:1002-16-0
- Empirical Formula: C5H11NO3
- Molecular Weight: 133.15
- MDL number: MFCD00059177
- EINECS: 213-684-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
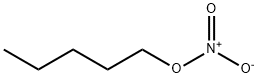
What is N-AMYL NITRATE?
Description
Amyl nitrate is a flammable, colorless liquidwith an ether-like odor. Molecular weight= 133.15; Boilingpoint=153°157℃; Melting/Freezing point= -123℃;Specific gravity= 1.0 at 20℃; Flash point= 48℃ (oc).Hazard Identification (based on NFPA-704 M RatingSystem): Health 2, Flammability 2, Reactivity 1, Oxidizer.Floats on water; insoluble.
Chemical properties
Colorless liquid; ethereal odor. Flammable.
Chemical properties
Amyl nitrate is a flammable, colorless liquid. Ethereal odor.
The Uses of N-AMYL NITRATE
Additive to increase cetane number of diesel fuels.
General Description
A clear colorless liquid with an ether-like odor. Flash point 118°F. About the same density as water and insoluble in water. Vapors heavier than air. Produces toxic oxides of nitrogen during combustion. Used as an additive for diesel fuels.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
N-AMYL NITRATE is an oxidizing reagent. When exposed to heat or flame N-AMYL NITRATE may decompose to form nitrogen oxides. Accelerates the burning of combustible materials. Can produce a violent reaction with reducing materials [Sax, 9th ed., p. 228].
Hazard
Oxidizing agent, moderate fire risk.
Health Hazard
Inhalation or ingestion may cause headache, methemoglobin, and nausea. Liquid and vapor irritate eyes. Contact with skin may cause slight irritation.
Safety Profile
Moderately toxic by inhalation. A flammable liquid. An oxidizing agent. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Highly flammable, Amyl nitrate is used as an ignition additive in diesel fuels.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine.
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trained onits proper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from heat,sparks, flames, and other combustible materials, such aswood, paper, or oil. Outside or detached storage is preferred.Before entering confined space where amyl nitrate may bepresent, check to make sure that an explosive concentrationdoes not exist. Metal containers involving the transfer of thischemical should be grounded and bonded. Where possible,automatically pump liquid from drums or other storage containers to process containers. Drums must be equipped withself-closing valves, pressure vacuum bungs, and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or stored ina manner that could create a potential fire or explosionhazard.
Shipping
UN1112 Amyl nitrate, Hazard Class: 3; Labels: 3-Flammable liquid.
Incompatibilities
A strong oxidizer. Violent reaction with many compounds, including reducing agents; chemically active metals; combustible materials, strong acids, alkaline earth sulfides, aluminum carbides, aluminum, amines, calcium sulfide, carbides, chlorine trifluoride, glycerin, hydrides, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, hydroxylamine, magnesium, metal powders, metal sulfides, molybdenum, phenylhydrazine, phosphorous red/ friction, phosphorous trichloride, silicon, sulfides, sulfur, sulfur dioxide, sulfur/friction, sulfuric acid, tungsten, hydrogen trisulfide. Temperature sensitive; may explode as temperature rises.
Waste Disposal
Incineration with scrubber to remove nitrogen oxides in effluent gases.
Properties of N-AMYL NITRATE
| Melting point: | -123.2°C |
| Boiling point: | 157 °C |
| Density | 1.00 |
| refractive index | 1.4120-1.4160 |
| Flash point: | 52 °C |
| Water Solubility | Insoluble in water |
| solubility | Chloroform (Soluble), Methanol (Sparingly) |
| form | Liquid |
| color | Colorless to Light yellow to Light orange |
| Dielectric constant | 9.0(18℃) |
| EPA Substance Registry System | Amyl nitrate (1002-16-0) |
Safety information for N-AMYL NITRATE
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P403+P235:Store in a well-ventilated place. Keep cool. P501:Dispose of contents/container to..… |
Computed Descriptors for N-AMYL NITRATE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![5-Amino-5-desoxy-1,4:3,6-dianhydro-D-glucit-2-nitrat-hydrochlorid [Ger man]](https://img.chemicalbook.in/CAS/GIF/81844-65-7.gif)

You may like
-
 Amyl Nitrate CAS 1002-16-0View Details
Amyl Nitrate CAS 1002-16-0View Details
1002-16-0 -
 Pentyl nitrate 95% CAS 1002-16-0View Details
Pentyl nitrate 95% CAS 1002-16-0View Details
1002-16-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1