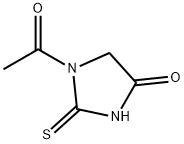
N-ACETYLTHIOUREA
- CAS NO.:591-08-2
- Empirical Formula: C3H6N2OS
- Molecular Weight: 118.16
- MDL number: MFCD00004937
- EINECS: 209-699-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
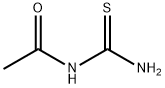
What is N-ACETYLTHIOUREA?
Chemical properties
white crystalline powder
Chemical properties
1-Acetyl-2-thiourea is a white crystalline solid; forming needles
The Uses of N-ACETYLTHIOUREA
N-Acetylthiourea is a derivative of Thiourea (T375450)
Definition
ChEBI: N-acetylthiourea is a member of the class of thioureas that is thiourea in which one of the hydrogens is replaced by an acetyl group. It is a member of acetamides and a member of thioureas. It is functionally related to a thiourea.
General Description
White crystalline solid. Noncombustible, but decomposes with heating.
Air & Water Reactions
Slightly soluble in water, soluble in hot water. Hydrolysis occurs rapidly with strong acid/base media.
Reactivity Profile
N-ACETYLTHIOUREA is incompatible with strong oxidizing agents, strong acids and strong bases. Decomposes when heated to give toxic oxides of sulfur and nitrogen.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Potential Exposure
Studied as possible rodenticide; used in organic synthesis.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Recrystallise the thiourea from AcOH; the solid is washed with Et2O and dried in air then at 100o. [Zahradnik Collect Czech Chem Commun 24 3678 1959, Beilstein 3 IV 354.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of N-ACETYLTHIOUREA
| Melting point: | 165-169 °C(lit.) |
| Density | 1.240 (estimate) |
| refractive index | 1.7000 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | 12.8g/l |
| form | Crystalline Powder |
| pka | 11.24±0.70(Predicted) |
| color | Pale cream |
| PH | 6.0 (173g/l, H2O, 20℃) |
| BRN | 969960 |
| CAS DataBase Reference | 591-08-2(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Acetyl-2-thiourea (591-08-2) |
Safety information for N-ACETYLTHIOUREA
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P309:IF exposed or if you feel unwell: P310:Immediately call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for N-ACETYLTHIOUREA
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 2-Hexyn-1-ol 98+View Details
2-Hexyn-1-ol 98+View Details
764-60-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
