N-ACETYL-4-BENZOQUINONE IMINE
Synonym(s):N-(4-Oxo-1-cyclohexa-2,5-dienylidene)acetamide;N-Acetyl-p-benzo-quinoneimine;NAPQI
- CAS NO.:50700-49-7
- Empirical Formula: C8H7NO2
- Molecular Weight: 149.15
- MDL number: MFCD00078909
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
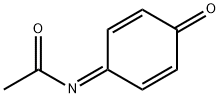
What is N-ACETYL-4-BENZOQUINONE IMINE?
Description
N-
Chemical properties
Yellow Crystalline Solid
The Uses of N-ACETYL-4-BENZOQUINONE IMINE
N-Acetyl-4-benzoquinone imine (NAPQI) is a cytochrome P450 3A4 and 2E1 metabolite of acetaminophen that can be toxic to the liver when acetaminophen is consumed in large doses. At therapeutic doses of acetaminophen, NAPQI is inactivated by conjugation with glutathione. However, following toxic doses of acetaminophen, available liver reserves of glutathione are quickly depleted due to clearance of excess NAPQI, which enables hepatic proteins to become covalently modified by reactive metabolites and eventually results in hepatocyte necrosis.
The Uses of N-ACETYL-4-BENZOQUINONE IMINE
A toxic metabolite of Acetaminophen, which reacts with serum proteins
The Uses of N-ACETYL-4-BENZOQUINONE IMINE
A toxic metabolite of Acetaminophen (A161220), which reacts with serum proteins.
The Uses of N-ACETYL-4-BENZOQUINONE IMINE
Reactant involved in:• ;Redox reactions1• ;Hydrohalogenation2• ;pH dependent reactions: in acidic media it is hydrolyzed, hydroxylated in alkaline media, and dimerized at intermediate pHs3• ;Mediation of acetaminophen hepatotoxicity4• ;Studies to identify the utility of acetaminophen for treating autoimmune disorders5
What are the applications of Application
N-Acetyl-4-benzoquinone Imine is a toxic metabolite of Acetaminophen
Definition
ChEBI: N-acetyl-1,4-benzoquinone imine is a quinone imine and a ketoimine. It is functionally related to a 1,4-benzoquinone imine.
Properties of N-ACETYL-4-BENZOQUINONE IMINE
| Melting point: | 74-75 |
| Boiling point: | 255.6±43.0 °C(Predicted) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| storage temp. | -70°C |
| solubility | Chloroform (Slightly, Sonicated), DMSO (Slightly) |
| form | Solid |
| pka | -3.37±0.20(Predicted) |
| color | Pale Yellow to Very Dark Red |
| Stability: | Temperature, Moisture, Light, and Oxygen Sensitive |
| CAS DataBase Reference | 50700-49-7 |
Safety information for N-ACETYL-4-BENZOQUINONE IMINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P362:Take off contaminated clothing and wash before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for N-ACETYL-4-BENZOQUINONE IMINE
Related products of tetrahydrofuran

You may like
-
 N-Acetylbenzoquinoneimine CAS 50700-49-7View Details
N-Acetylbenzoquinoneimine CAS 50700-49-7View Details
50700-49-7 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 99-30-9 99%View Details
99-30-9 99%View Details
99-30-9 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 98-56-6 99%View Details
98-56-6 99%View Details
98-56-6 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3