N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-aMine
Synonym(s):7-Nitro-N-(2-phenylphenyl)-2,1,3-benzoxadiazol-4-amine, Biphenyl-2-yl-(7-nitrobenzo[1,2,5]oxadiazol-4-ylamine, 10074-G5;Biphenyl-2-yl-(7-nitrobenzo[1,2,5]oxadiazol-4-yl)amine;c-Myc Inhibitor II - CAS 413611-93-5 - Calbiochem;N-[1,1′-Biphenyl-2-yl]-7-nitro-2,1,3-Benzoxadiazol-4-amine;N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-amine
- CAS NO.:413611-93-5
- Empirical Formula: C18H12N4O3
- Molecular Weight: 332.31
- MDL number: MFCD00576774
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:20
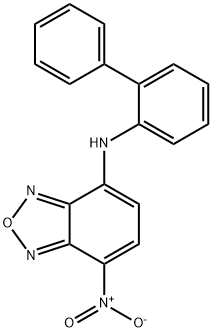
What is N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-aMine?
The Uses of N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-aMine
N-[1,1''-Biphenyl]-2-yl-7-nitro-2,1,3-benzoxadiazol-4-amine is a c-Myc inhibitor that suppresses its transcriptional activity.c-Myc is an oncoprotein often found overexpressed in various tumors.
What are the applications of Application
10074-G5 is an inhibitor of c-Myc-Max interaction
General Description
A cell-permeable benzoxadiazole compound that is shown to preferentially disrupt the interactions of c-Myc-Max, Mad1-Max, and Myf5-HEB, over those of 31 other pairs of HLH-, ZIP-, and HLH-ZIP-containing proteins. Similarly to 10058-F4 (Cat. No. 475956), 10074-G5 is shown to selectively inhibit the c-Myc-dependent growth of rat fibroblast cell line TGR1 and effectively suppress c-Myc-dependent transcription activity. Binding studies using various mutated and truncated c-Myc constructs reveal that 10074-G5 targets c-Myc helix-1 region between aa 363 and 381 (KD = 4.4 μM), while 10058-F4 interaction site is located within aa residues 402 through 412 (KD = 13 μM).
Biological Activity
10074-g5 is a c-myc inhibitor [1].c-myc is a bhlh-zip transcription factor involved in cell cycle progression, cellular growth and metabolism, differentiation, and apoptosis. overexpression of c-myc has been identified in numerous cancers, including prostate, pancreatic, lung, breast, and colon cancers, b-cell lymphoma, and leukemias. alterations in c-myc have been associated with cancer aggressiveness and poor treatment prognosis. inhibition of c-myc is an attractive pharmacological approach in the development of new anticancer treatments. inactivation of c-myc rapidly results in cell-cycle arrest, apoptosis, tumor vascular degeneration, redifferentiation of tumor cells, and ultimately tumor regression [1].the ic50 values of 10074-g5 against daudi cells and hl-60 cells were 15.6 ± 1.5 μm and 13.5 ± 2.1 μm, respectively. 10074-g5 (10 μm) inhibited c-myc/max dimerization and decreased total c-myc protein expression. in c.b-17 scid mice bearing daudi xenografts, treatment with 10074-g5, (20 mg/kg i.v., for 10 consecutive days) significantly inhibited tumor growth with no effects on body weight.
Biochem/physiol Actions
10074-G5 is a c-Myc/Max interaction inhibitor. The c-Myc oncoprotein and its partner Max are intrinsically disordered (ID) monomers that undergo coupled folding and binding upon heterodimerization. 10074-G5, similarly to 10058-F4 (#F3680), specifically inhibits this interaction by binding to c-Myc, thus preventing C-Myc specific DNA binding and target genes regulation. 10074-G5 (2.8 microM) is slightly more potent that 10058-F4 (5.2 microM). It was discovered that 10074-G5 binds to a different specific binding site (region) of C-Myc than 10054-F4. Thus, the compound may become desirable for probing different interactions.
References
[1] clausen d m, guo j, parise r a, et al. in vitro cytotoxicity and in vivo efficacy, pharmacokinetics, and metabolism of 10074-g5, a novel small-molecule inhibitor of c-myc/max dimerization[j]. journal of pharmacology and experimental therapeutics, 2010, 335(3): 715-727.
Properties of N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-aMine
| Boiling point: | 538.6±60.0 °C(Predicted) |
| Density | 1.408±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: >10mg/mL |
| pka | -2.06±0.50(Predicted) |
| form | powder |
| color | red |
Safety information for N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-aMine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-aMine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-(dimethylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-one Iminostilbene 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN Diclofenac Sodium SODIUM VALPROATE Racecadotril XANTHAN GUM 5-Fluoro-2-iodo-3-methylphenol 4-(Cyanomethyl)-1-piperidineacetamide 6-Bromo-2-iodo-4-methyl-3-pyridinamine 5-Amino-3-bromo-2-chlorobenzeneacetic acidYou may like
-
 10074-G5 CAS 413611-93-5View Details
10074-G5 CAS 413611-93-5View Details
413611-93-5 -
 1823754-06-8 98%View Details
1823754-06-8 98%View Details
1823754-06-8 -
 2-Chloro-5-(iodomethyl)pyrimidine 2268818-88-6 98%View Details
2-Chloro-5-(iodomethyl)pyrimidine 2268818-88-6 98%View Details
2268818-88-6 -
 2092793-98-9 98%View Details
2092793-98-9 98%View Details
2092793-98-9 -
 1360944-55-3 7-Methoxy-1H-indol-5-amine 98%View Details
1360944-55-3 7-Methoxy-1H-indol-5-amine 98%View Details
1360944-55-3 -
 2090480-14-9 98%View Details
2090480-14-9 98%View Details
2090480-14-9 -
![1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%View Details
1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%View Details
1651844-56-2 -
 176445-77-5 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 98+View Details
176445-77-5 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 98+View Details
176445-77-5