N-(2-Aminophenyl)pyrazine-2-carboxamide
Synonym(s):N-(2-Aminophenyl)pyrazine-2-carboxamide
- CAS NO.:926259-99-6
- Empirical Formula: C11H10N4O
- Molecular Weight: 214.22
- MDL number: MFCD09046162
- Update Date: 2024-11-15 19:19:13
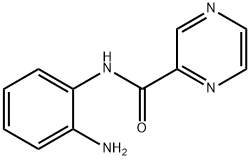
What is N-(2-Aminophenyl)pyrazine-2-carboxamide?
Description
BG45 is an HDAC class I inhibitor with selectivity for HDAC3 (IC50 = 289 nM). It inhibits HDAC1, HDAC2, and HDAC6 with greatly reduced potency (IC50s = 2, 2.2, and >20 μM, respectively). At concentrations up to 50 mg/kg, BG45 alone or in combination with bortezomib has been shown to dose-dependently inhibit tumor growth in a mouse model of multiple myeloma.
The Uses of N-(2-Aminophenyl)pyrazine-2-carboxamide
BG45 acts as an HDAC class I inhibitor with selectvity for HDAC3. HDACs represent novel targets for anti-tumer drugs treating various cancers including multiple myeloma cancer.
Definition
ChEBI: N-(2-aminophenyl)-2-pyrazinecarboxamide is an aromatic amide.
in vitro
bg45 is reported as an hdac class i inhibitor with selectivity for hdac3 over hdac1, 2. consistent with hdac3 knockdown data, bg45 significantly inhibited mm cell growth dose-dependently. importantly, bg45 also triggered a potent growth inhibitory effect against patient-derived mm cells, without affecting normal donor pbmcs [1].
in vivo
bg45 significantly inhibited mm tumor growth in a dose-dependent fashion. for example, significant differences were observed in control versus bg45 15 mg/kg, control versus bg45 50 mg/kg, and bg45 15 mg/kg versus bg45 50 mg/kg at day 22. moreover, bg45 50 mg/kg in combination with bortezomib enhanced either single agent activity further. these results confirmed that bg45 triggers in vivo anti-mm activities [1].
References
[1] minami j, suzuki r, mazitschek r, gorgun g, ghosh b, cirstea d, hu y, mimura n, ohguchi h, cottini f, jakubikova j, munshi nc, haggarty sj, richardson pg, hideshima t, anderson kc. histone deacetylase 3 as a novel therapeutic target in multiple myeloma. leukemia. 2014 mar;28(3):680-9.
Properties of N-(2-Aminophenyl)pyrazine-2-carboxamide
| Melting point: | 160-163°C |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
Safety information for N-(2-Aminophenyl)pyrazine-2-carboxamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-(2-Aminophenyl)pyrazine-2-carboxamide
New Products
(R)-3-Aminobutanenitrile Hydrochloride 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION SODIUM METHYL PARABEN Methylcobalamin (vitamin B12) Diclofenac Sodium SODIUM VALPROATE Racecadotril XANTHAN GUM 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidYou may like
-
 BG45 >95% CAS 926259-99-6View Details
BG45 >95% CAS 926259-99-6View Details
926259-99-6 -
 3-Bromo-2-hydroxybenzeneacetic acid 98%View Details
3-Bromo-2-hydroxybenzeneacetic acid 98%View Details
88491-46-7 -
 1805639-70-6 98%View Details
1805639-70-6 98%View Details
1805639-70-6 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 25408-95-1 98%View Details
25408-95-1 98%View Details
25408-95-1 -
 1-(3-Chloro-2-hydroxyphenyl)-2-propanone 98%View Details
1-(3-Chloro-2-hydroxyphenyl)-2-propanone 98%View Details
1784294-80-9 -
 99903-60-3 98%View Details
99903-60-3 98%View Details
99903-60-3 -
 5-Fluoro-2-iodo-3-methylaniline 1823368-42-8 98%View Details
5-Fluoro-2-iodo-3-methylaniline 1823368-42-8 98%View Details
1823368-42-8