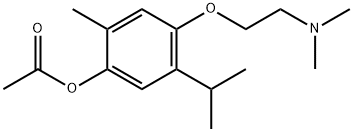
MOXISYLYTE
- CAS NO.:54-32-0
- Empirical Formula: C16H25NO3
- Molecular Weight: 279.37
- MDL number: MFCD00868265
- EINECS: 200-204-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
What is MOXISYLYTE?
Absorption
Moxisylyte is rapidly absorbed after oral administration. Its pharmacokinetic profile is linear in the dose range from 10 to 30 mg for the values of Cmax and AUC. After intravenous administration, the maximal plasma concentration was of 352.8 ng/ml with an AUC of 152.6 mcg h/L. In preclinical trials, the bioavailability was always presented in approximately 10%.
Toxicity
Moxisylyte is very well accepted by the patients and it presents very few adverse effects. The side effects are usually related to a profound alpha blockade than to a toxic response.
Originator
Carlytene,Dedieu,France,1962
The Uses of MOXISYLYTE
alpha-adrenergic blocker
Background
Moxisylyte, denominated as thymoxamine in the UK, is a specific and orally active α1-adrenergic antagonist. According to the WHO, moxisylyte is approved since 1987 and in the same year, it acquired the denomination of orphan product by the FDA. This drug was developed by the Japanese company Fujirebio and also by the American company Iolab in the late 80s.
Indications
By the WHO, moxisylyte is indicated for the symptomatic management of sequelae of cerebral infarction or hemorrhage. The cerebral infarction is characterized by the blockage of the artery either by the formation of a thrombus or an embolus.
On the other hand, the FDA classified moxisylyte for the reversal of phenylephrine-induced mydriasis in patients who have narrow anterior angles and are at risk of developing an acute attack of angle-closure glaucoma. Closed-angle glaucoma is caused by the contact between the iris and the trabecular meshwork. This contact will damage the aqueous outflow by the meshwork thus, increasing eye pressure and producing the symptoms of glaucoma. Mydriasis is referred to the dilatation of the pupils and this standard body function is known to be a trigger factor for the development of acute closed-angle glaucoma.This risk is explained by the generation of a pupillary block, which is the contact between the pupillary margins and the lens, thus preventing flow from the aqueous humor to the anterior chamber and followed by an increased pressure gradient.
Moxisylyte is also approved in France as the first drug for the treatment of impotence.
Definition
ChEBI: Acetic acid [4-[2-(dimethylamino)ethoxy]-2-methyl-5-propan-2-ylphenyl] ester is a monoterpenoid.
Manufacturing Process
A hydrochloric acid solution of 100 g of thymol in alcohol is reacted with 72 g
of sodium nitrite, the nitrosothymol (Organic Syntheses 6, New York, 1926, p.
92) thus obtained is introduced into ammonia, and is reduced by the
introduction of hydrogen sulfide to 4-aminothymol (Organic Syntheses Coll.
Vol. 1, New York, 1932, p. 458). 133.3 g of this 4-aminothymol are mixed
with 67 g of sodium acetate, 107 g of glacial acetic acid and 80 g of acetic
acid anhydride to form 4-acetaminothymol (Plancher, Gazzetta Chimica
Italiana 25, II, p. 388). 156 parts by weight of this last formed substance
dissolved in 600 cc of alcohol are added to a solution of 17.6 parts by weight
of sodium in 600cc of alcohol, the mixture being boiled under reflux for some
time with 82 g of dimethylaminoethyl chloride. The reaction product is treated
with water, and neutralized with hydrochloric acid using acid Congo reagent
indicator, and the alcohol is distilled off in vacuo. The base liberated by alkali
is dissolved in ether. By evaporating the ether solution the dimethylaminoethyl
ether of the 4-acetaminothymol is obtained as a brownish-yellow oil. After
some time this oil solidifies in a crystalline state.
100 g of this base are dissolved in a mixture of 300 cc of concentrated
hydrochloric acid (density 1.19) and 400 cc of water, and the solution is boiled
for one hour under a reflux condenser. Thereupon it is made alkaline,
extracted with ether, and the ether is distilled off. 23.6 g of the 4-
aminothymoxyethyldimethylamine thus obtained are diazotized in the
presence of sulfuric acid at a temperature not exceeding 0°C using a solution
of 7.2 g of sodium nitrite in 70 cc of water, and the diazo compound is heated
to boiling point after the addition of 1 g of copper sulfate, until no further gas
is evolved. It is then made alkaline, and carbon dioxide is introduced. The
base is precipitated first in an oily state, and soon becomes crystalline. The 4-
oxythymoxyethyldimethylamine forms a neutral hydrochloride which is readily
soluble in water, and has a melting point of 174°C to 175.5°C.
36.8 g of 4-oxythymoxyethyldimethylamine are boiled for one hour on a water
bath with 160 cc of acetic anhydride and 17.5 cc of pyridine. After this period,
the solution is diluted with water, made alkaline, and the base is extracted
with ether and the ether distilled off. With acids, the base obtained forms
crystalline salts which are readily soluble in water. The hydrochloride melts
between 208°C and 210°C.
Therapeutic Function
Vasodilator
World Health Organization (WHO)
Moxisylyte was introduced in the late 1980s. It belongs to the group of a-adrenergic blocking agents. In France it is indicated for the treatment of manifestations of benign prostatic hypertrophy. It is also marketed in the UK at lower dosages for the treatment of peripheral vascular disorders (Raynaud's disease).
Pharmacokinetics
Administration of moxisylyte has shown to improve peripheral flow in occlusive arterial disease with little effect in blood pressure. There are reports of increases in cutaneous blood flow and skin temperature after local application of moxisylyte.
Metabolism
The pharmacokinetic profile of moxisylyte can make this drug to be considered as a prodrug as its biotransformation is very rapid. This drug gets rapidly hydrolyzed by pseudocholinesterase in plasma and tissues to give the major metabolite deacetyl-thymoxamine. This first metabolite is later demethylated by the cytochrome P450 monooxygenase system to form deacetyl-demethyl-thymoxamine. Both of this major metabolites are pharmacologically active. The pharmacokinetic studies with moxisylyte in urine and feces have shown the presence of 8 different metabolites, where two of them are highly polar and resistant to enzymatic hydrolysis. From this metabolites, it has been detected the sulfate and glucuronide conjugates of the major metabolites.
Properties of MOXISYLYTE
| storage temp. | 2-8°C |
Safety information for MOXISYLYTE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for MOXISYLYTE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
