Molnupiravir
- CAS NO.:2349386-89-4
- Empirical Formula: C13H19N3O7
- Molecular Weight: 329.31
- EINECS: 604-604-1
- Update Date: 2025-03-06 08:32:39
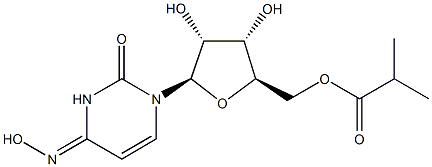
What is Molnupiravir?
Description
EIDD-2801 is an orally bioavailable prodrug of the antiviral nucleoside derivative N4- hydroxycytidine (NHC, EIDD-1931). It is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). The compound interferes with the action of viral RNA polymerase. It exerts its antiviral action through introduction of copying errors during viral RNA replication. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe. The broadspectrum antiviral agent EIDD-2801 inhibits viral RNA replication in various unrelated RNA viruses including influenza, Ebola, Venezuelan equine encephalitis virus (VEEV) and coronaviruses, including SARS-CoV, MERS-CoV and SARS-CoV-2. EIDD-2801 has the potential for COVID-19, seasonal and pandemic influenza treatment.
History
Molnupiravir (EIDD-2801/MK-4482) is an investigational, orally bioavailable form of a potent ribonucleoside analog that inhibits the replication of multiple RNA viruses including SARS-CoV-2, the causative agent of COVID-19. Molnupiravir has been shown to be active in several models of SARS-CoV-2, including for prophylaxis, treatment and prevention of transmission, as well as SARS-CoV-1 and MERS. EIDD-2801 was invented at Drug Innovations at Emory (DRIVE), LLC, a not-for-prot biotechnology company wholly owned by Emory University, and with partial funding support from the U.S. government. Since licensed by Ridgeback, all funds used for the development of EIDD-2801 by Ridgeback have been provided by Wayne and Wendy Holman and Merck.
The Uses of Molnupiravir
EIDD 2801 is an orally bioavailable broad-spectrum antiviral that inhibits SARS-CoV-2 and other multiple endemic, epidemic and bat coronavirus and has the potential for seasonal and pandemic influenza treatment. An isopropylester prodrug of the ribonucleoside analog of EIDD-1931 that exhibits anti-influenza virus and coronaviruses activities.
The Uses of Molnupiravir
EIDD 2801 is an orally bioavailable broad-spectrum antiviral that inhibits SARS-CoV-2 and other multiple endemic, epidemic and bat coronavirus and has the potential for seasonal and pandemic influenza treatment. An isopropylester prodrug of the ribonucleoside analog of EIDD-1931 that exhibits anti-influenza virus and coronaviruses activities.
Definition
Molnupiravir is a nucleoside analogue that is N(4)-hydroxycytidine in which the 5'-hydroxy group is replaced by a (2-methylpropanoyl)oxy group. It is the prodrug of the active antiviral ribonucleoside analog N(4)-hydroxycytidine (EIDD-1931), has activity against a number of RNA viruses including SARS-CoV-2, MERS-CoV, and seasonal and pandemic influenza viruses. It is currently in phase III trials for the treatment of patients with COVID-19. It has a role as a prodrug, an anticoronaviral agent and an antiviral drug. It is a nucleoside analogue, an isopropyl ester and a ketoxime. It derives from a N(4)-hydroxycytidine.
www.ebi.ac.uk
Biological Activity
EIDD-2801 is an orally active, 5′-isopropylester prodrug form of the antiviral ribonucleoside analog N4-hydroxycytidine (NHC, EIDD-1931) with known anti-viral activity against SARS-CoV, SARS-CoV-2, RSV, influenza A & B (IAV & IBV), HCV, pestivirus, bovine viral diarrhoea virus (BVDV), Ebola (EBOV), Chikungunya (CHIKV), venezuelan equine encephalitis (VEEV). EIDD-2801 is efficiently hydrolyzed to NHC in vivo, being more orally bioavailable than NHC in nonhuman primates and ferrets, while maintaining similar oral bioavailability as NHC in mice. EIDD-2801 displays in vivo efficacy against pandemic and seasonal IAV strains in ferrets (lowest ED = 2.3 and 7 mg/kg b.i.d. p.o., respectively).
Mode of action
Molnupiravir (development codes MK-4482 and EIDD-2801) is an investigational antiviral drug, which is the orally-bioavailable prodrug of the ribonucleoside analog N4-hydroxycytidine (NHC, EIDD-1931) that inhibits the replication of multiple RNA viruses, including SARS-CoV-2, by introducing copying errors during viral RNA replication. EIDD-1931 has broad spectrum antiviral activity against influenza virus and coronaviruses, such as MERS-CoV, and SARS-CoV.
Molnupiravir attacks the same viral enzyme as Gilead’s Remdesivir, but it can be taken orally. This would allow an administration at home and, therefore, earlier in the course of the disease. According to Ridgeback Biotherapeutics, Molnupiravir has an extremely high barrier to resistance.
According to MSD, Molnupiravir is aimed at the treatment of Covid-19 in
patients hospitalized due to mild, moderate or severe disease,
non-hospitalized patients with mild or moderate disease
Preparation and handling
Molnupiravir is supplied as a crystalline solid. A stock solution may be made by dissolving molnupiravir in the solvent of choice, which should be purged with an inert gas. Molnupiravir is soluble in organic solvents such as DMSO and dimethyl formamide. The solubility of molnupiravir in these solvents is approximately 30 mg/ml.
Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of molnupiravir can be prepared by directly dissolving the crystalline solid in aqueous buffers. The solubility of molnupiravir in PBS, pH 7.2, is approximately 1 mg/ml. We do not recommend storing the aqueous solution for more than one day.
Regulatory Status
Molnupiravir (as EIDD-2801) was developed at Drug Innovation Ventures at Emory (DRIVE), a not-forprofit biotechnology company owned by Emory University, Atlanta. It was then licensed by Ridgeback Biotherapeutics, and is now developed further in cooperation with Merck & Co. (in Europe: Merck Sharp & Dohme/ MSD).
Molnupiravir is not approved by the European Medicines Agency (EMA) or the American Food and Drug Administration (FDA).
References
[1] Toots M, Yoon J-J, Cox RM et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Science Translational Medicine 2019;11(515). Epub 23 Oct 2019.
[2] Toots M, Yoon J-J, Hart M et al. . Quantitative Efficacy Paradigms of the Influenza Clinical Drug Candidate EIDD-2801 in the Ferret Model. Transl Res 2020. Epub 2019 Dec 25.
[3] Halford B. An emerging antiviral takes aim at COVID-19. American Chemical Society; 2020 [14.12.2020]; Available from: https://cen.acs.org/pharmaceuticals/drug-development/emergingantiviral-takes-aim-COVID-19/98/web/2020/05.
[4] Ridgeback Biotherapeutics. OVERVIEW OF EIDD-2801 (MK-4482). [Presentation]. provided via email 2020.
Properties of Molnupiravir
| Melting point: | 156 - 159°C |
| storage temp. | -20°C, Inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| appearance | white to off-white solid |
| color | White to Off-White |
| InChI | InChI=1/C13H19N3O7/c1-6(2)12(19)22-5-7-9(17)10(18)11(23-7)16-4-3-8(15-21)14-13(16)20/h3-4,6-7,9-11,17-18,21H,5H2,1-2H3,(H,14,15,20)/t7-,9-,10-,11-/s3 |
Safety information for Molnupiravir
Computed Descriptors for Molnupiravir
| InChIKey | HTNPEHXGEKVIHG-JFPRWSRANA-N |
| SMILES | O[C@@H]1[C@@H]([C@@H](COC(=O)C(C)C)O[C@H]1N1C=C/C(=N/O)/NC1=O)O |&1:1,2,3,12,r| |
Molnupiravir manufacturer
SETV ASRV LLP
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran


![methyl 2-((1R,5S,6s)-3-azabicyclo[3.1.0]hexan-6-yl)acetate hydrochloride](https://img.chemicalbook.in/CAS/20200331/GIF/2102502-56-5.gif)
You may like
-
 2349386-89-4 99%View Details
2349386-89-4 99%View Details
2349386-89-4 -
 Molnupiravir 99%View Details
Molnupiravir 99%View Details -
 Molnupiravir 99%View Details
Molnupiravir 99%View Details -
 Molnupiravir 99%View Details
Molnupiravir 99%View Details -
 Molnupiravir 99%View Details
Molnupiravir 99%View Details -
 MOLNUPIRAVIR 98%View Details
MOLNUPIRAVIR 98%View Details -
 Molnupiravir 2349386-89-4 95-99 %View Details
Molnupiravir 2349386-89-4 95-99 %View Details
2349386-89-4 -
 EIDD-2801 CAS 2349386-89-4View Details
EIDD-2801 CAS 2349386-89-4View Details
2349386-89-4
