Doravirine
- CAS NO.:1338225-97-0
- Empirical Formula: C17H11ClF3N5O3
- Molecular Weight: 425.75
- MDL number: MFCD22417167
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-21 15:59:28
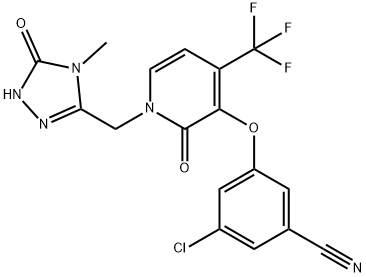
What is Doravirine?
Absorption
The absolute bioavailability of doravirine is 64% with a Tmax of 2 hours. Following oral [14C]doravirine administration, all of the administered dose was recovered and the agent is considered to be well absorbed. Moreover, its co-administration with food did not greatly alter doravirine's pharmacokinetic profile during clinical studies.
Toxicity
No clinically significant difference on the pharmacokinetics of doravirine were observed based on age (18 to 78 years of age), sex, and race/ethnicity, mild to severe renal impairment (creatinine clearance (CLcr) >15 mL/min, estimated by Cockcroft-Gault), or moderate hepatic impairment (Child-Pugh B). The pharmacokinetics of doravirine in patients with end-stage renal disease or undergoing dialysis, severe hepatic impairment (Child-Pugh C), or <18 years of age is unknown.
No adequate human data are available to establish whether or not doravirine poses a risk to pregnancy outcomes.
It is unknown whether doravirine is present in human milk, affects human milk production, or has effects on the breastfed infant. Because of the potential for (1) HIV-1 transmission (in HIV-negative infants), (2) developing viral resistance (in HIV positive infants), and (3) serious adverse reactions in a breastfed infant, instruct mothers not to breastfeed if they are receiving doravirine.
The safety and efficacy of doravirine have not been established in pediatric patients less than 18 years of age.
Clinical trials of doravirine did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration of doravirine in elderly patients, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of comorbidities or other drug therapy.
No dosage adjustment of doravirine is required in patients with mild, moderate, or severe renal impairment. Doravirine has not been adequately studied in patients with end-stage renal disease and has not been studied in dialysis patients.
No dosage adjustment of doravirine is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. Doravirine has not been studied in patients with severe hepatic impairment (Child-Pugh Class C).
Doravirine was not carcinogenic in long-term oral carcinogenicity studies in mice and rats at exposures up to 6 and 7 times, respectively, the human exposures at the RHD. A statistically significant incidence of thyroid parafollicular cell adenoma and carcinoma seen only in female rats at the high dose was within the range observed in historical controls.
Doravirine was not genotoxic in a battery of in vitro or in vivo assays, including microbial mutagenesis, chromosomal aberration in Chinese hamster ovary cells, and in in vivo rat micronucleus assays.
There were no effects on fertility, mating performance or early embryonic development when doravirine was administered to rats at systemic exposures (AUC) approximately 7 times the exposure in humans at the RHD.
The Uses of Doravirine
Doravirine is a novel non-nucleoside inhibitor of HIV-1 reverse transcriptase with potent activity against wild-type virus.
Background
Doravirine is an HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI) intended to be administered in combination with other antiretroviral medicines. Doravirine is available by itself or as a combination product of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg).
Doravirine is formally indicated for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment experience, further expanding the possibility and choice of therapeutic treatments available for the management of HIV-1 infection.
Indications
Doravirine is indicated, in combination with other antiretroviral agents, for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment history. It is also indicated to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen with no history of treatment failure and no known substitutions associated with resistance to doravirine.
Pharmacokinetics
In a clinical phase 2 trial evaluating a dose range of 0.25-2x the recommended dose of doravirine (in combination with emtricitabine/tenofovir) in HIV-1 infected subjects with no antiretroviral treatment history, no exposure-response relationship for efficacy was identified for doravirine.
Furthermore, at a dose of 1200 mg, which provides approximately 4 times the peak concentration observed following the recommended dose, doravirine does not prolong the QT interval to any clinically relevant extent.
Metabolism
Following absorption, unchanged parent drug is the major circulating component in plasma. Its M9 metabolite - a product of cytochrome P450 3A4/5 mediated oxidative metabolism - is the most abundant doravirine metabolite in the circulation.
Properties of Doravirine
| Melting point: | >278°C (dec.) |
| Density | 1.56±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 8.04±0.20(Predicted) |
| color | White to Off-White |
Safety information for Doravirine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Doravirine
Doravirine manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateYou may like
-
 1338225-97-0 Doravirine 98%View Details
1338225-97-0 Doravirine 98%View Details
1338225-97-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8