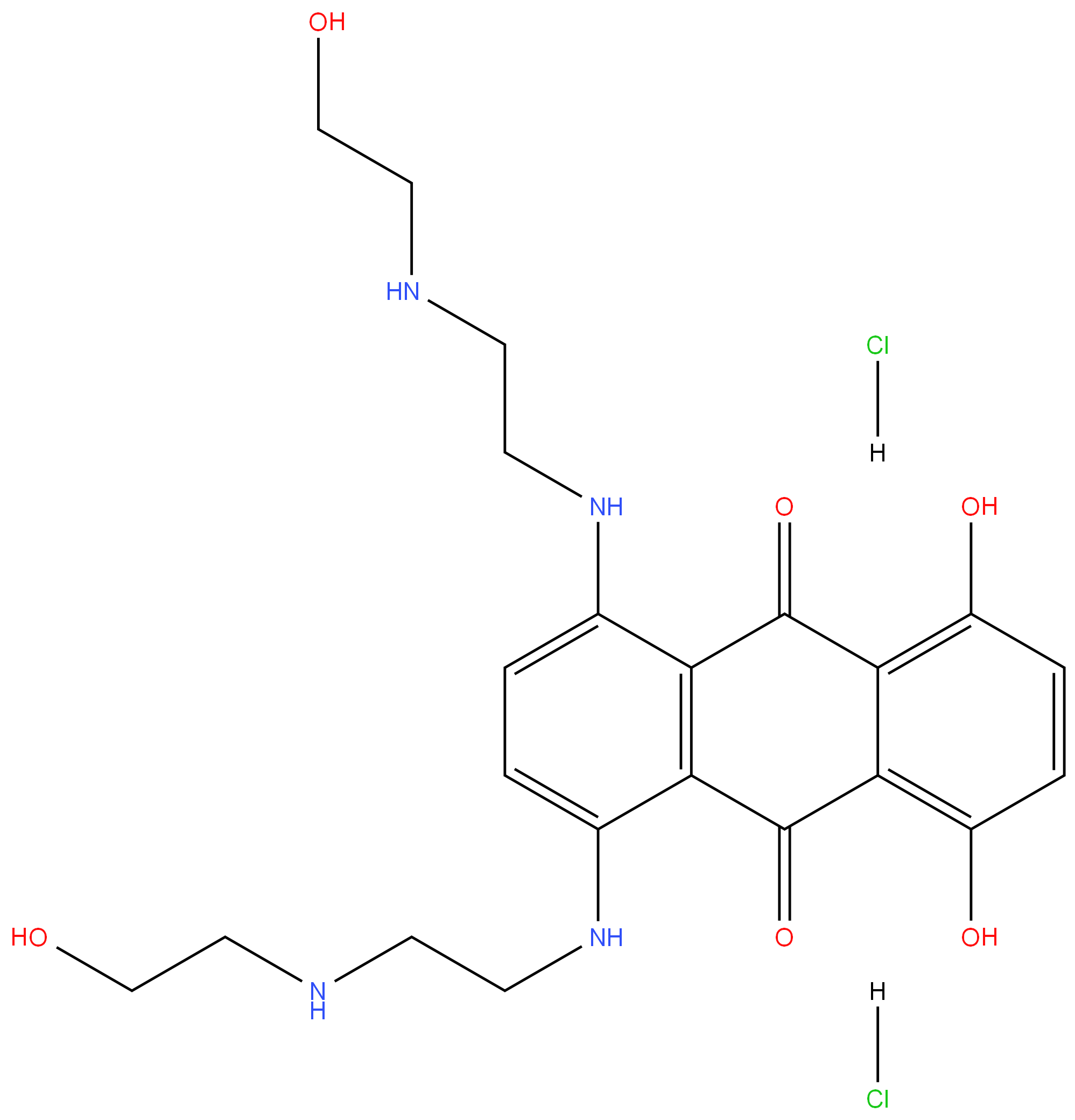
Mitoxantrone hydrochloride
Synonym(s):1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-9,10-anthracenedione dihydrochloride;Mitoxantrone dihydrochloride
- CAS NO.:70476-82-3
- Empirical Formula: C22H30Cl2N4O6
- Molecular Weight: 517.4028
- MDL number: MFCD00242943
- EINECS: 274-619-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
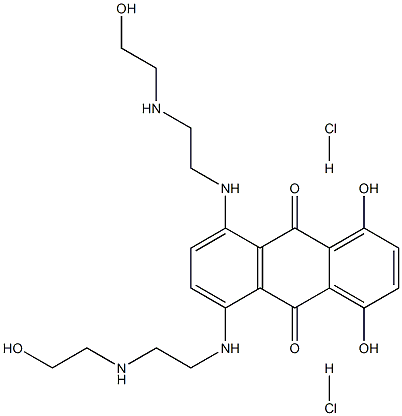
What is Mitoxantrone hydrochloride?
Description
Mitoxantrone hydrochloride is the first of the synthetic anthracenediones related to doxorubicin to reach the marketplace. Mitoxantrone is useful in the treatment of advanced localized and metastatic mammary carcinomas. It is reported to be less cardiotoxic than doxorubicin.
Description
Mitoxantrone is an anthraquinone that intercalates in DNA and inhibits topoisomerase II (IC50 = 5.3 μM), thus inhibiting cell proliferation. It also inhibits HIV-
Chemical properties
Dark blue, electrostatic, hygroscopic powder.
Originator
Lederle (USA)
The Uses of Mitoxantrone hydrochloride
analgesic, antipyretic
The Uses of Mitoxantrone hydrochloride
Mitoxanthrone hydrochloride USP (Novantrone) is used to traet acute nonlymphocytic leukemia, including myelogenous promyelocytic, monocytic, and erythroid acute leukemias.
The Uses of Mitoxantrone hydrochloride
Mitoxantrone dihydrochloride is an antiviral, antibacterial, antiprotozoal, immunomodulating, and antineoplastic cytostatic anthraquinone derivative. Induces DNA damage by intercalating into DNA and inhibiting Topo II (topoisomerase II). Mitoxantrone dihydrochloride induces interstrand DNA cross-links and DNA-protein cross-links in cellular systems. Mitoxantrone dihydrochloride has recently been shown to be an inhibitor of DNA methylation.
What are the applications of Application
Mitoxantrone Dihydrochloride is induces DNA damage by intercalating into DNA and inhibiting Topo II
Definition
ChEBI: Mitoxantrone dihydrochloride is a hydrochloride. It has a role as an antineoplastic agent. It contains a mitoxantrone.
Manufacturing Process
A suspension of 12.5 g of 2-(2-aminoethylamino)ethanol in 40 ml of
N,N,N',N'-tetramethylethylenediamine is stirred and de-aerated by bubbling
nitrogen in for 15 min. A 10.97 g of leuco-1,4,5,8-tetrahydroxyanthraquinone
is gradually added with stirring. The suspension is heated and stirred under
nitrogen at 50-52°C for 5 hours. The mixture is allowed to stand and cool
under nitrogen for 12 hours. The solid is collected by decantation, macerated
in ethanol, collected and washed with ethanol giving 15.06 g of the desired
product leuco-1,4-bis[2-(2-hydroxyethylamino)ethylamino]-5,8-
dihydroxyanthraquinone as a green-gray solid, melting point 129-131°C.
Chloranil oxidation. To 17.86 g of a suspension of the leuco-1,4-bis[2-(2-
hydroxyethylamino)ethylamino]-5,8-dihydroxyanthraquinone (0.03 mole) in 2-
methoxyethanol was added gradually with stirring 15 ml of 8 N ethanolic
hydrogen chloride. The system was chilled with an ice bath and stirred as 7.50
g (0.0305 mole) of chloranil powder was gradually added. The mixture was
stirred overnight at room temperature and diluted with 600 ml of ether. The
solid was collected and washed with tetrahydrofuran. Yield of 1,4-bis[2-(2-
hydroxyethylamino)ethylamino]-5,8-dihydroxyanthraquinone dihydrochloride
21.34 g, melting point 203-205°C (without recrystallisation).
brand name
Novantrone (Serono).
Therapeutic Function
Antineoplastic
General Description
Mitoxantrone is supplied as a blue aqueous solution in 10-and 20-mg vials for IV administration in the treatment of acute lymphoid leukemia, acute myeloid leukemia, breastcancer, prostate cancer, non-Hodgkin’s lymphoma, andmultiple sclerosis. The mechanisms of resistance are thesame as those seen for the anthracyclines. The distributionhalf-life is 1.1 to 3.1 hours, and the drug has a large volumeof distribution (11 L/kg). The elimination half-life rangedfrom 23 to 215 hours, and elimination was primarily via thebile. Metabolism of the agent involves oxidation of the sidechainalcohols to give the monocarboxylic and dicarboxylicacids.Other toxicities are those seen for the anthracyclinesand include myelosuppression, nausea, vomiting, mucositis,diarrhea, and alopecia. The intense color of the parent drugand metabolites may turn the urine blue.
Biological Activity
Mitoxantrone hydrochloride (Mitoxantrone dihydrochloride) is a type II DNA topoisomerase inhibitor. Disrupts DNA synthesis and repair and induces damage by DNA cross-linking. Also inhibits PIM1 kinase (IC50 = 51 nM). Immunomodulatory, antineoplastic and chemotherapeutic agent. Also USP11 inhibitor (IC50= 3.15 μM). Induces cell death of pancreatic cancer cell lines expressing wild-type BRCA2.
Biochem/physiol Actions
Mitoxantrone is a cytostatic anthracenedione that intercalates in DNA and increases the incidence of double-strand breaks by stabilizing the cleavable complex of topoisomerase II and DNA. Mitoxantrone also displays broad immunosuppressive activity inhibiting proliferation of all classes of lymphocytes and inducing apoptosis of antigen-presenting T cells. It used clinically as a chemotherapeutic agent against leukemias and solid tumors and as an immune system modulator in multiple sclerosis.
Clinical Use
Mitoxantrone is used in combination with other agents during the initial treatment of acute nonlymphocytic leukemia and hormone-refractory prostate cancer. Recent studies have shown that mitoxantrone also decreases the rate of relapse and disease progression in patients with multiple sclerosis. Although too toxic for use in patients with primary progressive disease, it is available for the treatment of chronic progressive, progressive relapsing, or deteriorating relapsing-remitting multiple sclerosis.
Side Effects
Common side effects of Mitoxantrone hydrochloride include: Swelling of the body; Infection, possibly in the blood, especially when white blood cell count is low; Sores in mouth and throat which may cause difficulty swallowing; Diarrhea, nausea, vomiting; Headache; Pain in belly, back, joints, or muscles; Abnormal or absence of menstrual period; Tiredness, fever; Hair loss; Hives, rash. Less common side effects include: Damage to the heart or heart failure which may cause shortness of breath, tiredness and swelling; Bruising, bleeding; Anemia which may cause tiredness, or may require transfusion; Liver damage which may cause yellowing of eyes and skin, swelling; Internal bleeding which may cause belly pain, black tarry stool, blood in vomit; Constipation, loss of appetite; Abnormal sexual function; Worry, depression; Swelling and redness at the site of the injection; Changes in weight; Swelling and redness of the whites of the eye; Increased sweating; Loss of nails, darkening of the skin and nails; Bluish/greenish discoloration of the urine, skin, eyes and saliva. A rare side effect is bone marrow cancer (leukaemia) caused by chemotherapy.
Metabolism
Mitoxantrone excretion primarily is biliary. Both the unchanged drug and inactive metabolites resulting from N-dealkylation, deamination, and oxidation of the resultant aldehyde to the carboxylic acid are observed. Both arms of the structure can be metabolized, leading to mono- or dicarboxylic acid metabolites, which are excreted as the glucuronide conjugate. The conjugated metabolites are an intense, dark blue in color and will result in blue-green urine.
Storage
Desiccate at RT
Properties of Mitoxantrone hydrochloride
| Melting point: | 203-205 C |
| storage temp. | 2-8°C |
| solubility | Sparingly soluble in water, slightly soluble in methanol, practically insoluble in acetone. |
| form | neat |
| form | Solid |
| color | Dark Blue to Black |
| Water Solubility | Soluble to 5 mM in water and to 75 mM in DMSO |
| InChI | InChI=1S/C22H28N4O6.2ClH/c27-11-9-23-5-7-25-13-1-2-14(26-8-6-24-10-12-28)18-17(13)21(31)19-15(29)3-4-16(30)20(19)22(18)32;;/h1-4,23-30H,5-12H2;2*1H |
| CAS DataBase Reference | 70476-82-3(CAS DataBase Reference) |
| EPA Substance Registry System | Mitoxantrone hydrochloride (70476-82-3) |
Safety information for Mitoxantrone hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H340:Germ cell mutagenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Mitoxantrone hydrochloride
| InChIKey | ZAHQPTJLOCWVPG-UHFFFAOYSA-N |
| SMILES | C1(O)=C2C(C(=O)C3=C(C2=O)C(NCCNCCO)=CC=C3NCCNCCO)=C(O)C=C1.[H]Cl.[H]Cl |
Mitoxantrone hydrochloride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 70476-82-3 Mitoxantrone hydrochloride 98%View Details
70476-82-3 Mitoxantrone hydrochloride 98%View Details
70476-82-3 -
 Mitoxantrone Dihydrochloride CAS 70476-82-3View Details
Mitoxantrone Dihydrochloride CAS 70476-82-3View Details
70476-82-3 -
 Mitoxantrone Dihydrochloride CAS 70476-82-3View Details
Mitoxantrone Dihydrochloride CAS 70476-82-3View Details
70476-82-3 -
 Mitoxantrone dihydrochloride 95.00% CAS 70476-82-3View Details
Mitoxantrone dihydrochloride 95.00% CAS 70476-82-3View Details
70476-82-3 -
 Mitoxantrone dihydrochloride CAS 70476-82-3View Details
Mitoxantrone dihydrochloride CAS 70476-82-3View Details
70476-82-3 -
 Mitoxantrone hydrochloride CAS 70476-82-3View Details
Mitoxantrone hydrochloride CAS 70476-82-3View Details
70476-82-3 -
 Mitoxantrone hydrochloride CAS 70476-82-3View Details
Mitoxantrone hydrochloride CAS 70476-82-3View Details
70476-82-3 -
 Minoxidil Related Compound E CAS 70476-82-3View Details
Minoxidil Related Compound E CAS 70476-82-3View Details
70476-82-3
