METYRAPONE
Synonym(s):2-Methyl-1,2-di-3-pyridyl-1-propanone;Metyrapone;Su-4885
- CAS NO.:54-36-4
- Empirical Formula: C14H14N2O
- Molecular Weight: 226.27
- MDL number: MFCD00006397
- EINECS: 200-206-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
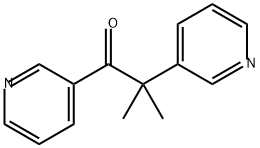
What is METYRAPONE?
Absorption
Absorbed rapidly and well when administered orally. Peak plasma concentrations are usually reached 1 hour after administration.
Toxicity
Oral LD50 in rats is 521 mg/kg. One case has been recorded in which a 6-year-old girl died after two doses of Metopirone, 2 g. Symptoms of overdose include cardiac arrhythmias, hypotension, dehydration, anxiety, confusion, weakness, impairment of consciousness, nausea, vomiting, epigastric pain, and diarrhea.
Description
Metyrapone (54-36-4) inhibits cytochrome P450-mediated prostaglandin omega hydroxylation. Inhibits steroid 11β-hydroxylase thereby inhibiting cortisol biosynthesis.
Originator
Metopirone,Ciba,US,1961
The Uses of METYRAPONE
Metyrapone acts as a glucocorticoid synthesis inhibitor, maintaining stress-hormone levels.
The Uses of METYRAPONE
Diagnostic aid (pituitary function);steroid 11?-hydroxylase inhibitor
Background
An inhibitor of the enzyme steroid 11-beta-monooxygenase. It is used as a test of the feedback hypothalamic-pituitary mechanism in the diagnosis of cushing syndrome.
Indications
Used as a diagnostic drug for testing hypothalamic-pituitary ACTH function. Occasionally used in Cushing's syndrome.
What are the applications of Application
Metyrapone is an inhibitor of cytochrome P450-mediated ω/ω-1 hydroxylase activity and CYP11B1
Definition
ChEBI: Metyrapone is an aromatic ketone that is 3,3-dimethylbutan-2-one in which the methyl groups at positions 1 and 4 are replaced by pyridin-3-yl groups. A steroid 11beta-monooxygenase (EC 1.14.15.4) inhibitor, it is used in the diagnosis of adrenal insufficiency. It has a role as a diagnostic agent, an antimetabolite and an EC 1.14.15.4 (steroid 11beta-monooxygenase) inhibitor.
Manufacturing Process
According to US Patent 2,966,493, the 2,3-bis-(3-pyridyl)-2,3-butanediol used
as the starting material may be prepared as follows. A solution of 1,430 g of
3-acetyl-pyridine in 7,042 ml of a 1 N aqueous solution of potassium
hydroxide is placed into a cathode chamber containing a mercury cathode with
a surface of 353 cm2 and is separated from an anode chamber by an Alundum
membrane. As anode a platinum wire is used and the anolyte consists of a 1
N solution of aqueous potassium hydroxide which is replenished from time to
time.
The electrolysis is carried out at a reference potential of -2.4 volts vs a
standard calomel electrode. An initial current density of 0.0403 amp/cm2 is
obtained which drops to 0.0195 amp/cm2 at the end of the reduction, which is
carried on over a period of 1,682 minutes at 15° to 20°C. The catholyte is
filtered, the solid material is washed with water and dried. 430 g of the 2,3-
bis-(3-pyridyl)-butane-2,3-diol is recrystallized from water, MP 244° to 245°C.
A mixture of 3.43 g of 2,3-bis-(3-pyridyl)-2,3-butane-diol and 25 ml of
concentrated sulfuric acid is heated to 76°C and kept at that temperature for
7? hours. It is then poured on ice, neutralized with 50% aqueous solution of
sodium hydroxide and the pH is adjusted to 8 with solid sodium carbonate.
The aqueous solution is three times extracted with ethyl acetate, the
separated organic layer dried over sodium sulfate and evaporated to dryness.
The residue is distilled and 1.86 g of viscous, colorless oil is obtained which is
purified by distillation. BP 140° to 160°C/0.07 mm. The infrared spectrum
shows the presence of a mixture of two compounds, one containing a
conjugated, the other one an unconjugated carbonyl group, without the
presence of a compound containing a hydroxyl group; thus the rearrangement
has taken place.
The resulting mixture does not crystalize and is converted into a mixture of
oximes by treatment of a solution of the mixture in 20 ml of ethanol with a
solution of 1.8 g of hydroxylamine sulfate in 3 ml of water. 1.8 g of sodium
acetate in 5 ml of water is added, and the mixture is refluxed for 5 hours,
then extracted with ethyl acetate, and the ethyl acetate solution is washed
with a saturated aqueous sodium chloride solution and dried over sodium
sulfate. After evaporating the solvent, the residue is triturated with warm
ether and 1.1 g of a crystalline oxime is obtained, MP 168° to 171°C.
0.1 g of the resulting oxime is dissolved in 5 ml of 2 N aqueous sulfuric acid
and the mixture is refluxed for 3 hours and allowed to stand overnight. After
being rendered basic by adding a concentrated aqueous solution of sodium
hydroxide and adjusted to a pH of 8 with sodium carbonate, the mixture is
extracted 3 times with ethyl acetate; the organic layer is washed with water,
dried and evaporated. Upon distillation of the residue an oily product is
obtained, BP 130° to 160°C/0.3 mm. Infrared analysis shows the presence of
a uniform compound, containing a conjugated carbonyl group. The 2-methyl-
1,2-bis-(3-pyridyl)-propane-1-one crystallizes upon standing at room
temperature or by covering the oily distillate with pentane and cooling to -
80°C and filtering the oily crystals. It melts after recrystallization from a
mixture of ether, hexane and petroleum ether at 48° to 50°C.
brand name
Metopirone Ditartrate (Ciba-Geigy).
Therapeutic Function
Diagnostic aid (pituitary function)
Biological Activity
Cytochrome P450 inhibitor. Blocks glucocorticoid synthesis via inhibition of steroid 11- β hydroxylase (CYP11B1) activity (IC 50 = 7.83 μ M). Also inhibits CYP3A4 and cytochrome P450-mediated ω / ω -1 hydroxylase activity.
Mechanism of action
Metyrapone (Metopirone) produces its primary pharmacological effect by inhibiting 11-β-hydroxylase, thereby causing diminished production and release of cortisol. The resulting reduction in the negative feedback of cortisol on the hypothalamus and pituitary causes an increase in corticotrophin release and in the secretion of precursor 11-deoxysteroids.
Pharmacokinetics
Metopirone is an inhibitor of endogenous adrenal corticosteroid synthesis.
Clinical Use
Metyrapone is used in the differential diagnosis of both
adrenocortical insufficiency and Cushing’s syndrome
(hypercortisolism). The drug tests the functional competence
of the hypothalamic–pituitary axis when the
adrenals are able to respond to corticotrophin; that is,
when primary adrenal insufficiency has been ruled out.
After metyrapone administration, a patient with a
disease of pituitary origin cannot achieve a compensatory
increase in the urinary excretion of 17-hydroxycorticosteroids
or 11-deoxysteroids. Moreover, if pituitary
corticotrophin is suppressed by an autonomously secreting
adrenal carcinoma, there will be no increase in response
to metyrapone. On the other hand, if pituitary
corticotrophin secretion is maintained, as occurs in adrenal
hyperplasia, the inhibition of corticoid synthesis produced
by metyrapone will stimulate corticotrophin secretion
and the release of metabolites of precursor urinary
steroids, which can be measured as 17-hydroxycorticosteroids.
Metyrapone is now used less frequently in the
differential diagnosis of Cushing’s syndrome because of
the ability to measure plasma corticotrophin directly.
The steroid-inhibiting properties of metyrapone
have also been used in the treatment of Cushing’s syndrome,
and it remains one of the more effective drugs
used to treat this syndrome. However, the compensatory
rise in corticotrophin levels in response to falling
cortisol levels tends to maintain adrenal activity.This requires
that glucocorticoids be administered concomitantly
to suppress hypothalamic–pituitary activity.
Although metyrapone interferes with 11β- and 18-
hydroxylation reactions and thereby inhibits aldosterone
synthesis, it may not cause mineralocorticoid deficiency
because of the compensatory increased production
of 11-desoxycorticosterone.
Side Effects
Side effects associated with the use of metyrapone include gastrointestinal distress, dizziness, headache, sedation, and allergic rash. The drug should not be used in cases of adrenocortical insufficiency or when hypersensitivity reactions can be expected. When administered to pregnant women during the second or third trimesters, the drug may impair steroid biosynthesis in the fetus. Because metyrapone is relatively nontoxic, it is used in combination therapy with the more toxic aminoglutethimide to reduce its dosage.
Metabolism
Hepatic. The major biotransformation is reduction of the ketone to metyrapol, an active alcohol metabolite. Metyrapone and metyrapol are both conjugated with glucuronide.
Storage
Store at +4°C
Properties of METYRAPONE
| Melting point: | 52-55 °C(lit.) |
| Boiling point: | 367.89°C (rough estimate) |
| Density | 1.0852 (rough estimate) |
| refractive index | 1.6419 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: soluble (sparingly) |
| form | solid |
| pka | 4.61±0.10(Predicted) |
| color | White |
| Merck | 13,6181 |
| BRN | 163023 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
| EPA Substance Registry System | Metyrapone (54-36-4) |
Safety information for METYRAPONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for METYRAPONE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Metyrapone CAS 54-36-4View Details
Metyrapone CAS 54-36-4View Details
54-36-4 -
 2-Methyl-1,2-di-3-pyridyl-1-propanone CAS 54-36-4View Details
2-Methyl-1,2-di-3-pyridyl-1-propanone CAS 54-36-4View Details
54-36-4 -
 Metyrapone CAS 54-36-4View Details
Metyrapone CAS 54-36-4View Details
54-36-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
