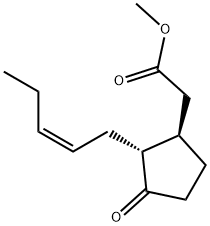
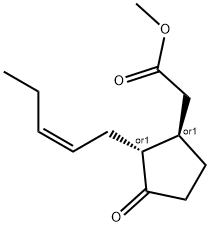
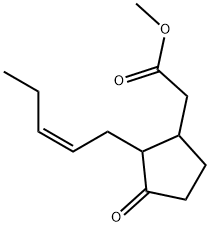
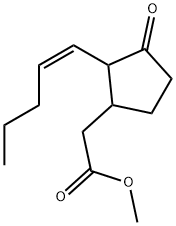
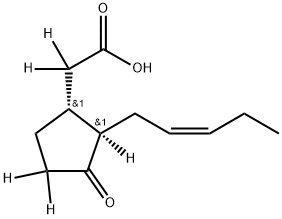
METHYL JASMONATE
Synonym(s):3-Oxo-2-(2-pentenyl)cyclopentaneacetic acid, methyl ester;Methyl 3-oxo-2-(2-pentenyl)cyclopentaneacetate
- CAS NO.:1211-29-6
- Empirical Formula: C13H20O3
- Molecular Weight: 224.3
- MDL number: MFCD00151382
- EINECS: 214-918-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is METHYL JASMONATE?
Description
Methyl jasmonate has a powerful, floral-herbaceous, sweet, persistent odor. Can be isolated from jasmine oil; or it may be prepared synthetically (probably in the trans-form) from muconic acid via the methyl-3-oxo-cyclopenthyl acetate.
Chemical properties
METHYL JASMONATE is a volatile component of jasmine flower absolute. It is
a colorless to pale yellow liquid, bp0.125 kPa 116–118 °C, d20
20 1.022–1.028, n20
D
1.473–1.477, possessing an odor reminiscent of the floral heart of jasmine.
Synthesis of the title compound is accomplished by reaction of (2Z)-2-buten-1-yl
bromide with Li in the presence of copper-(I) iodide, and the product is treated
with a mixture of 2-methylene- and 4-methylene-3-oxocyclopentylacetic acid
methyl ester. The aforementioned ester mixture is obtained by reaction of methyl
3-oxocyclopentylacetate with formaldehyde.
An alternative route uses a palladium-catalyzed decarboxylation of the readily
available substituted allyl 2-oxocyclopentanecarboxylates, which leads to the
corresponding cyclopentenones. Addition of dimethylmalonate, with subsequent
saponification/decarboxylation and, if necessary, (Z)-selective hydrogenation,
yields methyl jasmonate:
Methyl jasmonate exists in an equilibrium mixture in which the trans-isomer
dominates. But of all possible four enantiomeric isomers, chiefly the minor (+)-cis-isomer is responsible for the typical sensory properties of methyl jasmonate.
Therefore, several approaches to obtain this material selectively have been developed
either by directed syntheses or by enrichment using
special isomerization distillation techniques.
Methyl jasmonate is used in fine fragrances where it provides rich, soft effects in jasmine and muguet compositions.
Chemical properties
Methyl jasmonate has a powerful, floral–herbaceous, sweet, persistent odor.
Occurrence
Reportedly identified in jasmine oil (Jasminum grandiflorum L.) and in Tunisian rosemary. Also found in lemon peel oil, peppermint oil and green and fermented tea
The Uses of METHYL JASMONATE
Methyl ester in perfumes.
The Uses of METHYL JASMONATE
(-)-Jasmonic Acid Methyl Ester can be used in biological study where it can exert concn.-dependent effect on SE1 and upregulated SE1 expression in both young and mature leaves of Gynostemma pentaphyllum, with greater upregulation in young leaves than in mature leaves.
Definition
ChEBI: A jasmonate ester that is the methyl ester of jasmonic acid.
Aroma threshold values
Detection: 5.7 ppm
Taste threshold values
Taste characteristics at 15 ppm: floral, fruity, green, waxy, seedy and melon-like
Trade name
Jasmoneige® (Nippon Zeon), Splendione® (Firmenich)
Synthesis
Can be isolated from jasmine oil; synthetically it can be prepared (probably in the trans-form) from muconic acid via the methyl-3-oxo-cyclopentyl acetate
Properties of METHYL JASMONATE
| Boiling point: | 110 °C0.2 mm Hg(lit.) |
| alpha | D -76.5° (c = 3.4 in CH3OH) |
| Density | 1.03 g/mL at 25 °C(lit.) |
| FEMA | 3410 | METHYL JASMONATE |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Almost insoluble in water, soluble in alcohol and oils. |
| form | oily liquid |
| Specific Gravity | 1.02 |
| color | Colorless |
| Odor | at 100.00 %. floral fresh petal magnolia oily waxy |
| JECFA Number | 1400 |
| CAS DataBase Reference | 1211-29-6(CAS DataBase Reference) |
| EPA Substance Registry System | Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-pentenyl-, methyl ester, (1R,2R)- (1211-29-6) |
Safety information for METHYL JASMONATE
Computed Descriptors for METHYL JASMONATE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![L-Isoleucine, N-[2-[(1R,2S)-3-oxo-2-(2Z)-2-penten-1-ylcyclopentyl]acetyl]-](https://img.chemicalbook.in/CAS/20200611/GIF/145414-77-3.gif)
![N-[(-)-JASMONOYL]-(S)-ISOLEUCINE (JaIle)](https://img.chemicalbook.in/CAS/20180808/GIF/120330-93-0.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1