METERGOLINE
Synonym(s):[(8β)-1,6-Dimethylergolin-8-yl)methyl]carbamic acid phenylmethyl ester;N-CBZ-[(8β)-1,6-Dimethylergolin-8-yl]methylamine
- CAS NO.:17692-51-2
- Empirical Formula: C25H29N3O2
- Molecular Weight: 403.52
- MDL number: MFCD00153829
- EINECS: 241-686-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 16:00:36
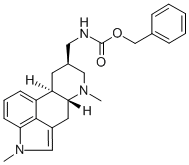
What is METERGOLINE?
Description
Metergoline (17692-51-2) is an ergot alkaloid derivative acting as antagonist at serotonin 5HT1 and 5HT2 receptors1,2 and 5HT7 receptors3. A safe and effective agent for management of hyperprolactinemic amenorrhea and anovulation.4 Displays a long-lasting antidepressant effect in seasonal affective disorder.5
Chemical properties
White Solid
Originator
Liserdol,Farmitalia,Italy,1970
The Uses of METERGOLINE
Metergoline is an antagonist at 5-HT1, 5-HT2, and 5-HT7 serotonin receptors. Metergoline is an analgesic; antipyretic.
Background
Metergoline is an ergot-derived psychoactive drug that acts as a ligand for serotonin and dopamine receptors. Metergoline is an antagonist at various 5-HT receptor subtypes at a relatively low concentration and agonist at dopamine receptors. Its use has been studied in various clinical settings such as a treatment for seasonal affective disorder, prolactin hormone regulation due to its inhibitory effect on prolactin release, premenstrual dysphoric disorder in women and antianxiety treatment.
What are the applications of Application
Metergoline is an antagonist at 5-HT1, 5-HT2, and 5-HT7 serotonin receptors
Definition
ChEBI: Metergoline is an ergoline alkaloid that is the N-benzyloxycarbonyl derivative of lysergamine. A 5-HT2 antagonist. Also 5-HT1 antagonist and 5-HT1D ligand. Has moderate affinity for 5-HT6 and high affinity for 5-HT7. It has a role as a serotonergic antagonist, a dopamine agonist and a geroprotector. It is an ergoline alkaloid and a carbamate ester.
Manufacturing Process
16 g of 1-methyl-dihydro-lysergamine (the 10-position hydrogen has the αconfiguration) are dissolved in 80 cc of anhydrous pyridine by mildly heating. To the solution, cooled to -10°C and stirred, 18 cc of 85% carbobenzoxychloride (in toluene) diluted in 36 cc of chloroform are added dropwise, rather rapidly. The mixture is kept at -10°C during the addition, and for 10 minutes afterwards. The cooling means is removed and the temperature is allowed to rise to room level in 10 minutes. The reaction mixture is diluted with 240 cc of chloroform and rapidly washed with 80 cc of 5% aqueous sodium hydroxide solution, with saturated aqueous sodium bicarbonate solution, and finally with water.
The chloroform solution is briefly dried over anhydrous sodium sulfate and evaporated to dryness in vacuo at 40°C. The oily residue is taken up in 160 cc of benzene and passed through a column containing 48 g of alumina. The column is then eluted with further 160 cc of benzene. The collected eluates are evaporated in vacuo at 40°C. The thick oily residue is mixed with a small amount of anhydrous diethyl ether. After some time a crystalline mass is obtained, which is collected and washed with a small amount of benzene and diethyl ether. 12 g of white crystals are obtained, melting at 146° to 148°C.
Therapeutic Function
Analgesic
Biological Activity
Antagonist at 5-HT 1 /5-HT 2 with activity at 5-HT 1D . Has moderate affinity for 5-HT 6 and high affinity for 5-HT 7 .
Biochem/physiol Actions
Antagonist at 5-HT1, 5-HT2, and 5-HT7 serotonin receptors; analgesic; antipyretic.
Metabolism
Not Available
storage
Room temperature
References
Aulakh et al. (1992), Effects of various serotonin receptor subtype-selective antagonists alone and on m-chlorophenylpiperazine-induced neuroendocrine changes in rats; J. Pharmacol. Exp. Ther., 263 588 Bagdy et al. (1992), Pharmacological characterization of serotonin receptor subtypes involved in vasopressin and plasma renin activity responses to serotonin agonists; Eur. J. Pharmacol., 210 285 Knight et al. (2009), Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, and other inactivating antagonists; Mol. Pharmacol., 75 374 Falsetti et al. (1982), Metergoline in the management of hyperprolactinemia amenorrhea and anovulation; Gynecol. Obstet. Invest., 13 108 Turner et al. (2002), Double-blind, placebo-controlled study of single-dose metergoline in depressed patients with seasonal affective disorder; J. Clin. Psychopharmacol., 22 216
Properties of METERGOLINE
| Melting point: | 148-150 °C(lit.) |
| Boiling point: | 612.5±55.0 °C(Predicted) |
| alpha | D28 -7 ±2° |
| Density | 1.25±0.1 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | 0.1 M HCl: 1.4 mg/mL |
| form | solid |
| pka | 12.45±0.46(Predicted) |
| color | White |
| Merck | 13,5962 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethaqnol may be stored at -20°C for up to 2 month. |
| CAS DataBase Reference | 17692-51-2(CAS DataBase Reference) |
Safety information for METERGOLINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for METERGOLINE
| InChIKey | WZHJKEUHNJHDLS-QTGUNEKASA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Metergoline CAS 17692-51-2View Details
Metergoline CAS 17692-51-2View Details
17692-51-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8