Metaldehyde
Synonym(s):Acetaldehyde polymerized
- CAS NO.:108-62-3
- Empirical Formula: C8H16O4
- Molecular Weight: 176.21
- MDL number: MFCD00071549
- EINECS: 203-600-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
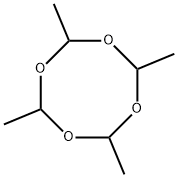
What is Metaldehyde?
Description
Metaldehyde (CAS: 108-62-3) is a polymer of acetaldehyde
that is used as a pesticide against snails and slugs, and also as
a fuel. It was discovered by von Liebig in 1835, and a century
later its use as a molluscicide was proposed by Gimingham and
Newton in 1937. It is manufactured by reacting acetaldehyde
with various acids at a low temperature. As a molluscicide,
metaldehyde is used for controlling slugs and snails in gardens
of a variety of vegetable and ornamental crops. For this purpose
it is available in both solid and liquid formulations with
metaldehyde concentrations ranging from 1.8 to 8% in pellet
form, or in concentrations of up to 20% in liquid formulations.
Some metaldehyde formulations may consist of other pesticides,
such as arsenic; while others may contain toxic solvents
like ethylene glycol. Therefore, a thorough forensic analysis of
the product is recommended to assess complete risk associated
with ingestion of these products.
Children under the age of 3 years and pets are most
commonly poisoned by ingestion of pellets or cakes containing
metaldehyde. Intoxication by metaldehyde is typically acute,
characterized initially by gastrointestinal and subsequently
followed predominantly by neurologic signs. Vomiting, diarrhea,
ataxia, tremors, convulsions, and hyperthermia are
among the most common toxic signs in children and animals.
Other clinical signs in children include mental confusion,
muscle cramps and tremors, loss of consciousness, and coma.
The proximate neurotoxic mechanisms are not known but
reduced levels of neurotransmitters such as serotonin and
g-aminobutryric acid (GABA) are implicated. Delayed effects of
acute exposure include hepatotoxicity characterized by hepatic
necrosis and increased serum liver enzymes. In general, males
metabolize metaldehyde twice as fast as females. Therefore,
female animals are more sensitive to metaldehyde poisoning
compared to males. Most of the neurotoxic signs are seen at
dose levels greater than 100 mg of metaldehyde per kilogram.
Developmental studies have shown that metaldehyde exposure
during pregnancy does not adversely affect fetuses.
Chronic metaldehyde exposure is unlikely. However,
experimental research has shown that metaldehyde is toxic
under chronic exposure conditions. In males, chronic metaldehyde
exposure causes testicular atrophy and is also toxic to
the prostate gland. It causes atrophy of the prostate gland. It is
not clear whether the effects on male gonads are due to
endocrine disruption or through a different mechanism. There
is also suggestive evidence of carcinogenic potential based on
the presence of benign tumors in female rats and mice of both
sexes.
Provided treatment is initiated early in cases of acute
exposure, prognosis is good. In dogs, the mortality rate in
a recently completed study was 16%. Because there is no
specific antidote, treatment consists of decontamination
measures and symptomatic therapy. Recent formulations of
metaldehyde pesticides contain denatonium benzoate,
a bittering agent. The purpose of adding the bittering agent
is to deter excessive ingestion of the products. This, along
with new labeling guidelines highlighting the risk to children
and pets has caused annual cases to drop significantly
since 2006.
Chemical properties
white fine crystalline powder
Chemical properties
Metaldehyde is a white crystalline powder with a mild menthol odor.
The Uses of Metaldehyde
There are over 50 pesticides containing metaldehyde registered for use in the United States. It is commonly used as a pesticide against slugs and snails. It is formulated in ready to use liquid, paste, granules, pellets, minipellets, or meal baits. It is also used as a camping fuel. It may be purchased in a tablet form for the latter use.
What are the applications of Application
2,4,6,8-Tetramethyl-1,3,5,7-Tetraoxacyclooctane is a compound that has been proposed as a potential pesticide
Definition
ChEBI: Metaldehyde is a member of the class of tetroxocanes that is 1,3,5,7-tetroxocane which carries four methyl groups at positions 2,4,6 and 8. It is a potent molluscicide and the active ingredient in most slug pellets used for crop protection. It has a role as a molluscicide and a fuel.
Flammability and Explosibility
Flammable
Agricultural Uses
Molluscicide: A tetramer of acetaldehyde, metaldehyde is a molluscicide used in a variety of vegetable and ornamental crops in the field or greenhouse. It may be formulated with or without calcium arsenate and is also available in a mixed formulation with thiram. A U.S. EPA restricted Use Pesticide (RUP).
Trade name
ANTIMILACE®; ARIOTOX®; CEKUMETA®; DEADLINE®; DURHAM®; HALIZAN®; LIMATOR®; META®; METASON®; NAMEKIL®; SLUG-TOX®; TRAILS END®
Safety Profile
Human poison by ingestion. Human systemic effects by ingestion: convulsions or effect on seizure threshold. Moderately toxic by inhalation and skin contact. Experimental reproductive effects. Mutation data reported. A flammable solid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.
Potential Exposure
It is used as a poison for slugs and snails, and as a fuel in small heaters.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. 1716 Metaldehyde If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Environmental Fate
Metaldehyde is of low persistence in the soil. It is degraded aerobically with a half-life of approximately 67 days to inactive residues. Metaldehyde is soluble in water. Metaldehyde undergoes rapid hydrolysis to acetaldehyde in an aquatic environment.
storage
Color Code—Red: Flammability Hazard: Store in a flammable storage area. Prior to working with metaldehyde you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, wellventilated area away from strong oxidizers. Where possible, automatically transfer material from drums or other storage containers to process containers. Drums must be equipped with self-closing valves, pressure vacuum bungs, and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical. Wherever this chemical is used, handled, manufactured, or stored, use explosion-proof electrical equipment and fittings.
Shipping
UN1332 Metaldehyde, Hazard Class: 4.1; Labels: 4.1-Flammable solid
Toxicity evaluation
The toxic mechanism in snails and slugs is different from that in mammals and birds. Metaldehyde irreversibly damages mucous secreting cells of snails and slugs on which these organisms depend. In animals and birds, the proximate toxic mechanism of metaldehyde is not known, but is either due to toxicity of metaldehyde itself or of its metabolite acetaldehyde on the brain and other tissues. The fundamental neurotoxic molecular mechanisms are not known, but a depression of serotonin and GABA is suspected to play a role.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides
Properties of Metaldehyde
| Melting point: | 246 °C |
| Boiling point: | 65°C/15mmHg(lit.) |
| Density | 1.27 |
| vapor pressure | 6.6Pa at 25℃ |
| refractive index | 1.4220 (estimate) |
| Flash point: | 50°C |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Fine Crystalline Powder |
| color | White |
| Water Solubility | 0.02 g/100 mL (20 ºC) |
| Sublimation | 112-115 ºC |
| CAS DataBase Reference | 108-62-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetaldehyde, tetramer(108-62-3) |
| EPA Substance Registry System | Metaldehyde (108-62-3) |
Safety information for Metaldehyde
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H228:Flammable solids H301:Acute toxicity,oral H313:Acute toxicity,dermal H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for Metaldehyde
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Metaldehyde, 97% CAS 108-62-3View Details
Metaldehyde, 97% CAS 108-62-3View Details
108-62-3 -
 Metaldehyde CAS 108-62-3View Details
Metaldehyde CAS 108-62-3View Details
108-62-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1