MERCUROUS BROMIDE
- CAS NO.:15385-58-7
- Empirical Formula: Br2Hg2
- Molecular Weight: 560.99
- MDL number: MFCD00053986
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-31 15:13:05
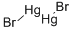
What is MERCUROUS BROMIDE?
Description
Mercury(I) bromide or mercurous bromide, Hg2Br2, is a compound that changes color from white to yellow when heated and fluoresces a salmon color under UV light. It is used in acousto-optical devices. A very rare mineral is kuzminite, Hg2(Br,Cl)2. Mercury(I) bromide is formed through the oxidation of elemental mercury with elemental bromine or via the addition of sodium bromide to a solution of mercury(I) nitrate.
Chemical properties
white, odorless, tasteless powder(s); tetr; sensitive to light (darkens); decomposed by hot HCl or alkali bromides; becomes yellow when heated, returns to white color when cooled; prepared by oxidation of Hg with Br2 or as a precipitate by addition of NaBr to HgNO3 solution [KIR81] [HAW93] [MER06]
General Description
White, odorless powder. Darkens on exposure to light. Sublimes at high temperatures.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
MERCUROUS BROMIDE is incompatible with the following: Acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper .
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Properties of MERCUROUS BROMIDE
| Melting point: | 445 °C(lit.) |
| Boiling point: | sublimes at 340–350℃ [HAW93] |
| Density | 7.31 g/mL at 25 °C(lit.) |
| solubility | insoluble in H2O, ethanol, ethyl ether |
| form | white tetragonal crystals or powder |
| color | white tetragonal crystals, crystalline or
powder |
| Water Solubility | g/L solution H2O: 3.9×10?5 (25°C) [KRU93]; insoluble alcohol, ether [MER06]; soluble in fuming HNO3, hot conc H2SO4 [HAW93] |
| Solubility Product Constant (Ksp) | pKsp: 22.19 |
| EPA Substance Registry System | Mercury, dibromodi-, (Hg-Hg) (15385-58-7) |
Safety information for MERCUROUS BROMIDE
Computed Descriptors for MERCUROUS BROMIDE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
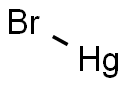
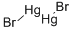
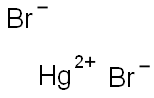
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4