MERCURIC ACETATE
Synonym(s):Mercuric acetate
- CAS NO.:1600-27-7
- Empirical Formula: C4H6HgO4
- Molecular Weight: 318.68
- MDL number: MFCD00012165
- EINECS: 216-491-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
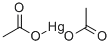
What is MERCURIC ACETATE?
Description
Mercury (II) acetate is the chemical compound with the formula Hg(O2CCH3)2. Commonly abbreviated Hg (OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors.
Chemical properties
Mercuric acetate, Hg(C2H3O2)2 , is a toxic, light-sensitive white powder, soluble in water,alcohol,and acetic acid. On exposure to heat, mercuric acetate produces toxic fumes of mercury/mercuric oxide. Mercuric acetate is incompatible with chromic acid, chromic anhydride, nitric acid, perchloric acid, permanganates, sodium peroxide, potassium hydroxide, hydrogen peroxides, acid anhydrides, and strong oxidising agents.
Chemical properties
Mercuric acetate is a white crystalline solid with a mild vinegar-like odor.
Physical properties
Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 ?. Three long, weak intermolecular Hg···O bonds of about 2.75 ? are also present,resulting in a slightly distorted square pyramidal coordination geometry at Hg.
The Uses of MERCURIC ACETATE
Mercuric acetate is used as an oxidizing agent in organic synthesis. It is used in oxymercuration of double bonds. Mercuric acetate is used in non-aqueous titration. It is employed in the manufacture of phenyl mercury compounds which have pharmaceutical applications. It removes the acetamidomethyl protecting group from protected thiol, and converts thiocarbonate esters into dithiocarbonates. It promotes the addition of hydroxide and alkoxide across carbon-carbon double bonds.
The Uses of MERCURIC ACETATE
Used in determination of nitrate in chromium compounds
The Uses of MERCURIC ACETATE
Chiefly for mercuration of organic compounds; for the absorption of ethylene.
Reactions
Arenes undergo "mercuration" upon treatment with Hg(OAc)2. The one acetate group that remains on mercury can be displaced by chloride :
C6H5OH + Hg(OAc)2 → C6H4(OH)-2-HgOAc + HOAc
C6H4(OH)-2-HgOAc + NaCl → C6H4(OH)-2-HgCl + NaOAc
The Hg2+ center binds to alkenes, inducing the addition of hydroxide and alkoxide. For example, treatment of methylacrylate with mercuric acetate in methanol gives an α - mercuri ester :
Hg(OAc)2 + CH2 = CHCO2CH3 + CH3OH → CH3OCH2CH(HgOAc)CO2CH3+ HOAc
Mercury(II) has a high affinity for sulfur ligands. Hg (OAc)2 can be used as a reagent to remove the acetamidomethyl protecting group, which is used to "protect" thiol groups in organic synthesis. Similarly Hg(OAc)2 is a standard reagent to convert thiocarbonate esters into dithiocarbonates:
(RS)2C=S + H2O + Hg(OAc)2 → (RS)2C=O + HgS + 2 HOAc
Mercury (II) acetate is used for oxymercuration reactions.
General Description
White crystalline solid with an odor of vinegar. Sensitive to light. Density 3.25 g / cm3. Toxic by inhalation (dust, etc.) and by ingestion.
Air & Water Reactions
Water soluble. Decomposed by water to form a yellow insoluble product.
Reactivity Profile
MERCURIC ACETATE is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, and copper .
Hazard
Toxic by ingestion, inhalation, and skin absorption; strong irritant.
Health Hazard
MERCURIC ACETATE may cause death by hypovolemic shock or kidney failure. Chronic exposure may lead to kidney failure.
Fire Hazard
When heated to decomposition, MERCURIC ACETATE emits toxic fumes of mercury. Avoid light.
Safety Profile
Poison by ingestion, intravenous, intraperitoneal, and subcutaneous routes. Moderately toxic by skin contact. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Hg. See also MERCURY COMPOUNDS.
Potential Exposure
Mercuric acetate is used chiefly for mercuration of organic compounds; for the absorption ofethylene; as a chemical intermediate for phenylmercuric acetate; a mildewcide; and other organomercury compounds. It is used as a catalyst in organic synthesis; and in the manufacture of pharmaceuticals.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Speed in removing material from skin is of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water or milk. Do not induce vomiting. Medical observation is recommended for 2448 h after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a corticosteroid spray. Antidotes and special procedures for medical personnel: The drug NAP (n-acetyl penicillamine) has been used to treat mercury poisoning, with mixed success. Note to physician: For severe poisoning BAL [British AntiLewisite, dimercaprol, dithiopropanol (C3H8OS2)] has been used to treat toxic symptoms of certain heavy metals poisoning including mercury. Although BAL is reported to have a large margin of safety, caution must be exercised, because toxic effects may be caused by excessive dosage. Most can be prevented by premedication with 1-ephedrine sulfate (CAS: 134-72-5).
Storage
Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with this chemical you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, wellventilated area away from oxidizers (such as perchlorates, peroxides, permanganates, chlorates and nitrates), light, heat, and acids.
Shipping
UN1629 Mercury acetate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Recrystallise it from glacial acetic acid. POISONOUS. [Beilstein 2 IV 114.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Light and heat can cause decomposition.
Properties of MERCURIC ACETATE
| Melting point: | 179-182 °C(lit.) |
| Density | 3,29 g/cm3 |
| vapor density | 11 (vs air) |
| storage temp. | Store at RT. |
| solubility | Water (Slightly) |
| form | Solid |
| color | White to almost white |
| Specific Gravity | 3.27 |
| Odor | Slight acetic acid odor |
| Water Solubility | Soluble in water. |
| Sensitive | Light Sensitive |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Merck | 14,5873 |
| BRN | 3563831 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. May decompose upon exposure to light. |
| CAS DataBase Reference | 1600-27-7(CAS DataBase Reference) |
| EPA Substance Registry System | Mercuric acetate (1600-27-7) |
Safety information for MERCURIC ACETATE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for MERCURIC ACETATE
MERCURIC ACETATE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Mercury(II) acetate CAS 1600-27-7View Details
Mercury(II) acetate CAS 1600-27-7View Details
1600-27-7 -
 Mercury(II) acetate CAS 1600-27-7View Details
Mercury(II) acetate CAS 1600-27-7View Details
1600-27-7 -
 Mercuric Acetate ACS CAS 1600-27-7View Details
Mercuric Acetate ACS CAS 1600-27-7View Details
1600-27-7 -
 Mercuric acetate 95% CAS 1600-27-7View Details
Mercuric acetate 95% CAS 1600-27-7View Details
1600-27-7 -
 mercury acetate CASView Details
mercury acetate CASView Details -
 Mercuric acetate, GR CAS 1600-27-7View Details
Mercuric acetate, GR CAS 1600-27-7View Details
1600-27-7 -
 Mercury(II) acetate, Purity: Min.97%, Grade: Lr/arView Details
Mercury(II) acetate, Purity: Min.97%, Grade: Lr/arView Details
1600-27-7 -
 MERCURIC ACETATE 98.5%View Details
MERCURIC ACETATE 98.5%View Details
1600-27-7
