Mavacamten
- CAS NO.:1642288-47-8
- Empirical Formula: C15H19N3O2
- Molecular Weight: 273.33
- MDL number: MFCD31746877
- EINECS: 213-161-7
- Update Date: 2025-12-17 20:37:47
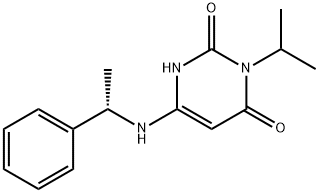
What is Mavacamten?
Absorption
Mavacamten has an estimated oral bioavailability of at least 85% and Tmax of 1 hour. Mavacamten exposures (AUC) increased up to 220% in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. The effect of severe (Child-Pugh C) hepatic impairment is unknown.
Toxicity
Human experience of overdose with CAMZYOS is limited. CAMZYOS has been given as a single dose of up to 144 mg in patients with HCM. One subject administered a single dose of 144 mg experienced serious adverse events including vasovagal reaction, hypotension, and asystole, but the subject recovered. In healthy subjects, doses of up to 25 mg have been administered for up to 25 days, with 3 of 8 participants treated at the 25-mg dose level experiencing 20% or greater reductions in LVEF. An infant's death was reported after accidental ingestion of three 15-mg capsules.
Systolic dysfunction is the most likely result of overdosage of CAMZYOS. Treatment of overdose with CAMZYOS consists of discontinuation of CAMZYOS treatment as well as medically supportive measures to maintain hemodynamic stability, including close monitoring of vital signs and LVEF and management of the clinical status of the patient. Overdose in humans can be life-threatening and result in asystole refractory to any medical intervention.
Mavacamten was not genotoxic in a bacterial reverse mutation test (Ames test), a human in vitro lymphocyte clastogenicity assay, or a rat in vivo micronucleus assay. There was no evidence of carcinogenicity seen in a 6-month rasH2 transgenic mouse study at mavacamten doses of up to 2.0 mg/kg/day in males and 3.0 mg/kg/day in females, which resulted in exposures (AUC) that were 1.8- and 3-fold in males and females, respectively, compared to AUC exposures in humans at the MRHD.
In reproductive toxicity studies, there was no evidence of the effects of mavacamten on mating and fertility in male or female rats at doses up to 1.2 mg/kg/day, or on the viability and fertility of offspring of dams dosed up to 1.5 mg/kg/day. Plasma exposure (AUC) of mavacamten at the highest dose tested was the same as in humans at the MRHD.
The safety of mavacamten has been evaluated in rats and dogs at multiple dose levels (0.06 to 10 mg/kg/day) orally. Noted toxicities, including echocardiographic findings, reduction in systolic function, cardiac dilation, and death, as well as increased heart weights in rats, were consistent with mavacamten’s mechanism of action and primary pharmacological activity. Other findings included cardiac osseous metaplasia in rats and QTc prolongation in dogs. Plasma exposures (AUC) at the NOAEL in rats and dogs were 0.1 and 0.3 times, respectively, human exposure (AUC) at the MRHD.
Description
Mavacamten(MYK-461)is a first-in-class allosteric inhibitor of cardiac myosin that promises to provide clinicians with targeted therapy for these patients. It is a mechanistically novel small molecule that acts on the sarcolemma to specifically inhibit contractility and has been proposed as a treatment for HCM.
The Uses of Mavacamten
MYK-461 is a drug used to treat obstructive hypertrophic cardiomyopathy as a cardiac myosin inhibitor. Mavacamten is used to treat adults with symptomatic obstructive hypertrophic cardiomyopathy. It is used to treat symptomatic obstructive hypertrophic cardiomyopathy (HCM).
Indications
Mavacamten is indicated for the treatment of adults with symptomatic New York Heart Association (NYHA) class II-III obstructive hypertrophic cardiomyopathy (HCM) to improve functional capacity and symptoms by the FDA, Health Canada, and the EMA.
Background
Mavacamten is a myosin inhibitor indicated for the treatment of adults with symptomatic New York Heart Association (NYHA) class II-III obstructive hypertrophic cardiomyopathy (HCM). It received initial US FDA approval in 2022, and it is one of the first myosin inhibitors to be used in humans. Mavacamten was also approved by Health Canada in October 2022 and by EMA in July 2023 for the same indication.
Mechanism of action
Mavacamten is thought to work by reducing cardiac muscle contractility by inhibiting excessive myosin-actin cross-bridge formation that results in hypercontractility, left ventricular hypertrophy and reduced compliance. In clinical and preclinical studies, it has consistently reduced biomarkers of cardiac wall stress, lessened excessive cardiac contractility, and increased diastolic compliance.
Pharmacokinetics
Mavacamten is a myosin inhibitor to prevent muscle hypercontractility. It binds to myosin and inhibits myosin interaction with actin at various stages of the thermomechanical cycle. Mechanistic studies show that mavacamten can inhibit myosin in both its active and relaxed form, thus effectively alleviating excess sarcomere power, a hallmark of hypertrophic cardiomyopathy.
In the EXPLORER-HCM trial, patients achieved reductions in mean resting and provoked (Valsalva) LVOT gradient by Week 4 which were sustained throughout the 30-week trial. At Week 30, the mean (SD) changes from baseline in resting and Valsalva LVOT gradients were -39 (29) mmHg and -49 (34) mmHg, respectively, for the CAMZYOS group and -6 (28) mmHg and -12 (31) mmHg, respectively, for the placebo group. The reductions in the Valsalva LVOT gradient were accompanied by decreases in LVEF, generally within the normal range. Eight weeks after discontinuation of CAMZYOS, mean LVEF and Valsalva LVOT gradients were similar to baseline.
Echocardiographic measurements of the cardiac structure showed a mean (SD) reduction from baseline at Week 30 in left ventricular mass index (LVMI) in the mavacamten group (-7.4 [17.8] g/m2) versus an increase in LVMI in the placebo group (8.9 [15.3] g/m2). There was also a mean (SD) reduction from baseline in left atrial volume index (LAVI) in the mavacamten group(-7.5 [7.8] mL/m2) versus no change in the placebo group (-0.1 [8.7] mL/m2). The clinical significance of these findings is unknown.
A reduction in a biomarker of cardiac wall stress, NT-proBNP, was observed by Week 4 and sustained through the end of treatment.
At Week 30 compared with baseline, the reduction in NT-proBNP after mavacamten treatment was 80% greater than for placebo (proportion of geometric mean ratio between the two groups, 0.20 [95% CI: 0.17, 0.24]). The clinical significance of these findings is unknown.
In healthy volunteers receiving multiple doses of mavacamten, a concentration-dependent increase in the QTc interval was observed at doses up to 25 mg once daily. No acute QTc changes have been observed at similar exposures during single-dose studies. The mechanism of the QT prolongation effect is not known. A meta-analysis across clinical studies in HCM patients does not suggest clinically relevant increases in the QTc interval in the therapeutic exposure range. In HCM, the QT interval may be intrinsically prolonged due to the underlying disease, in association with ventricular pacing, or in association with drugs with the potential for QT prolongation commonly used in the HCM population. The effect of coadministration of mavacamten with QT-prolonging drugs or in patients with potassium channel variants resulting in a long QT interval has not been characterized.
Side Effects
Common side effects include:Dizziness, fainting, Incidence not known, Chest pain or tightness, decreased urine output, dilated neck veins, irregular breathing, irregular heartbeat, swelling of the face, fingers, feet, or lower legs, trouble breathing, unusual tiredness or weakness and weight gain.
Metabolism
Mavacamten is extensively metabolized, primarily through CYP2C19 (74%), CYP3A4 (18%), and CYP2C9 (8%).
Properties of Mavacamten
| Density | 1.19±0.1 g/cm3(Predicted) |
| solubility | insoluble in H2O; ≥11.32 mg/mL in EtOH with ultrasonic; ≥13.65 mg/mL in DMSO |
| form | solid |
| pka | 8.83±0.40(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C15H19N3O2/c1-10(2)18-14(19)9-13(17-15(18)20)16-11(3)12-7-5-4-6-8-12/h4-11,16H,1-3H3,(H,17,20)/t11-/m0/s1 |
Safety information for Mavacamten
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P330:Rinse mouth. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Mavacamten
| InChIKey | RLCLASQCAPXVLM-NSHDSACASA-N |
| SMILES | C1(=O)NC(N[C@H](C2=CC=CC=C2)C)=CC(=O)N1C(C)C |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 1642288-47-8 Mavacamten 98%View Details
1642288-47-8 Mavacamten 98%View Details
1642288-47-8 -
 1642288-47-8 98%View Details
1642288-47-8 98%View Details
1642288-47-8 -
 1642288-47-8 98%View Details
1642288-47-8 98%View Details
1642288-47-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6