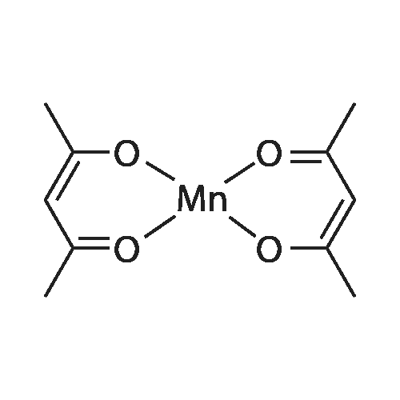
MANGANESE(II) ACETYLACETONATE
Synonym(s):2,4-Pentanedione manganese(II) derivative;Bis(acetylacetonato)manganese(II), Bis(2,4-pentanedionato)manganese(II);Manganese(II) acetylacetonate;Manganous acetylacetonate;Mn(acac)2
- CAS NO.:14024-58-9
- Empirical Formula: C10H14MnO4
- Molecular Weight: 253.15
- MDL number: MFCD00000022
- EINECS: 237-858-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-16 17:17:29
What is MANGANESE(II) ACETYLACETONATE?
Chemical properties
Beige solid
General Description
Manganese(II) acetylacetonate (Mn(acac)2) is a transition metal acetylacetonate that is trimeric in hydrocarbon solvents. It is monomeric and tetrahedral in its vapor state. It shows Mn-O bond energy of 65 kcal mol-1. It can be used with potassium permanganate in preparation of manganese triad (Mn(acac)3).
Safety Profile
Questionable carcinogen with experimental neoplastigenic and tumorigenic data. When heated to decomposition it emits acrid smoke and irritating fumes. See also iMANGANESE COMPOUNDS.
Purification Methods
Purify it by stirring 16g of reagent for a few minutes with 100mL absolute EtOH and filter by suction as rapidly as possible through coarse filter paper. Sufficient EtOH is added to the filtrate, to make up for the loss of EtOH and to redissolve any solid that separates. Water (15mL) is added to the filtrate, and the solution is evaporated with a stream of N2 until reduced to half its volume. Cool for a few minutes and filter off the yellow crystals, dry them under a stream of N2, then in a vacuum at room temperature for 6-8hours. These conditions are important for obtaining the dihydrate. A vacuum to several mm of Hg or much lower pressure for several days produces the anhydrous complex. The degree of hydration can be established by determining the loss in weight of 100g of sample after heating for 4hours at 100o and <20mmHg. The theoretical loss in weight for 2H2O is 12.5%. The material sublimes at 200o/2mm. It is soluble in heptane, MeOH, EtOH or *C6H6 at 30o. [Charles Inorg Synth VI 164 1960, Fernelius & Biswas Inorg Synth V 105 1957, Beilstein 3 II 3122.]
Properties of MANGANESE(II) ACETYLACETONATE
| Melting point: | 248-250 °C (dec.)(lit.) |
| Boiling point: | 130.3°C |
| Density | 1,6 g/cm3 |
| storage temp. | Store below +30°C. |
| solubility | 11.5g/l |
| form | Powder |
| Specific Gravity | 1.60 |
| color | tan |
| Water Solubility | Soluble in water |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 4157965 |
| Exposure limits | OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| Stability: | hygroscopic |
| EPA Substance Registry System | Manganese, bis(2,4-pentanedionato-.kappa.O,.kappa.O')- (14024-58-9) |
Safety information for MANGANESE(II) ACETYLACETONATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for MANGANESE(II) ACETYLACETONATE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Bis(2,4-pentanedionato)manganese(ii) dihydrate 95% CAS 14024-58-9View Details
Bis(2,4-pentanedionato)manganese(ii) dihydrate 95% CAS 14024-58-9View Details
14024-58-9 -
 Manganese(II) acetylacetonate CAS 14024-58-9View Details
Manganese(II) acetylacetonate CAS 14024-58-9View Details
14024-58-9 -
 Manganese(II) acetylacetonate CAS 14024-58-9View Details
Manganese(II) acetylacetonate CAS 14024-58-9View Details
14024-58-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
