Magnesium diamide
- CAS NO.:7803-54-5
- Empirical Formula: H4MgN2
- Molecular Weight: 56.35
- MDL number: MFCD01940560
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:56
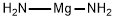
What is Magnesium diamide?
Chemical properties
Whitish to gray crystals. D 1.40. Decomposes when heated.
The Uses of Magnesium diamide
Polymerization catalyst.
General Description
A whitish to gray-colored crystalline solid. Denser than water. Contact may irritate skin, eyes and mucous membranes. Vapors may be toxic by inhalation. Used to make other chemicals.
Air & Water Reactions
May spontaneously ignite upon exposure to air. Soluble in water. Reacts violently with water to form caustic ammonia/ammonium hydroxide and heat.
Reactivity Profile
Bases or alkalis are chemically similar to sodium hydroxide (NaOH) or sodium oxide (Na2O). They neutralize acids exothermically to form salts plus water. When soluble in water they give solutions having a pH greater than 7.0. Mixing these materials with water can generate troublesome amounts of heat as the base is dissolved or diluted. Bases react with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. Bases may initiate polymerization reactions in polymerizable organic compounds, especially epoxides). They may generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. Materials of this group often serve as catalysts.
Hazard
A pyrophoric material igniting in air at room temperature. Evolves ammonia on vigorous reaction with water.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Properties of Magnesium diamide
| Melting point: | decomposes when heated [HAW93] |
| Density | d425 1.39 |
| solubility | reacts with H2O |
| form | white powder |
| color | white powder; flammable |
| Water Solubility | reacts violently with H2O releasing NH3 [MER06] |
| EPA Substance Registry System | Magnesium amide (Mg(NH2)2) (7803-54-5) |
Safety information for Magnesium diamide
Computed Descriptors for Magnesium diamide
New Products
4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIRRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 1714107-48-8 5-Iodo-3-methyl-2-nitropyridine 98%View Details
1714107-48-8 5-Iodo-3-methyl-2-nitropyridine 98%View Details
1714107-48-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9