Lysergic acid diethylamide
Synonym(s):Lysergic acid diethylamide
- CAS NO.:50-37-3
- Empirical Formula: C20H25N3O
- Molecular Weight: 323.44
- MDL number: MFCD00135795
- EINECS: 200-033-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
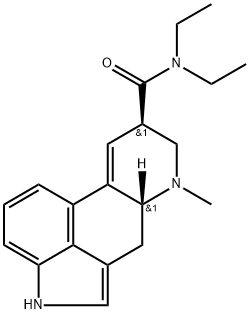
What is Lysergic acid diethylamide?
Description
Lysergic acid diethylamide (LSD) is synthetically derived from the fungus, Claviceps purpuria. Several isomers of this compound exist, although only the D-isomer is considered active. LSD was first synthesized by Albert Hofmann in 1938 in a search for an analeptic drug. After finding its psychogenic activity, it was marketed by Sandoz laboratories under the name Delysid?. It was also reportedly used by the Central Intelligence Agency as a tool for interrogation. Due to its potential for ‘bad trips,’ ‘flashbacks,’ and potential risk for brain injury, LSD was banned federally in 1966 and is currently only used illicitly. LSD is chemically similar to other naturally occurring lysergamides found in multiple species of morning glory.
Description
LSD is familiar to anyone who remembers the 1960s. It is formally known as lysergic acid diethylamide or lysergide, but during its heyday it was simply called “acid”.
A. Stoll and A. Hofmann synthesized LSD in 1943 from ergotamine, which was derived from the grain fungus ergot. Hofmann also discovered its psychedelic properties, and for a while LSD was investigated as a psychotherapeutic drug. Its binding to serotonin receptors causes its psychedelic effects.
But along came the 1960s and Timothy Leary, who urged young people to take an acid “trip” to “turn on, tune in, and drop out”. The drug became widely abused; and in 1968, the US government made its possession illegal. Since that time, it has had little psychiatric use; but recently research into its uses has increased.
Chemical properties
crystalline solid
History
lysergic acid diethylamide(LSD) is a hallucinogenic drug that was first synthesized a Swiss scientist in the 1930s. During the Cold War, the CIA conducted clandestine experiments with LSD (and other drugs) for mind control, information gathering and other purposes. Over time, the drug became a symbol of the 1960s counterculture, eventually joining other hallucinogenic and recreational drugs at rave parties.
The Uses of Lysergic acid diethylamide
Labelled Lysergide (L488010)
The Uses of Lysergic acid diethylamide
Potent hallucinogen; non-selective serotonin receptor agonist. Has been used experimentally as adjunct in study and treatment of mental disorders. Controlled substance.
The Uses of Lysergic acid diethylamide
LSD is obtained by partial synthesis fromD-lysergic acid. It is also produced bymicrobial reaction of Claviceps paspali overthe hydroxylethylamide. It is a well-knownhallucinogen and a drug of abuse, listed asa controlled substance in the U.S. Code ofFederal Regulations (Title 21, Part 1308.11,1987). It is used as an antagonist to serotoninand in the study and treatment of mentaldisorders.
Definition
ChEBI: An ergoline alkaloid arising from formal condensation of lysergic acid with diethylamine.
General Description
Prismatic crystals (from benzene). Tasteless and odorless. A hallucinogen.
Reactivity Profile
An amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Health Hazard
LSD is a strong psychedelic agent. Theeffects in human are excitement, euphoria,hallucinations, and distorted perceptions.It alters the thinking process, producingillusions and loss of contact with reality.In humans, a dose (intramuscular)of 0.7–0.9 mg/kg or an oral dose of2.5–3.0 mg/kg may produce the effectsabove. Other symptoms may include nausea,vomiting, dilation of pupils, restlessness, andperipheral vascoconstriction. However, thereis no reported case of overdose death. In rabbits,somnolence, ataxia, and an increase inbody temperature were the symptoms notedat the LD50 (intravenous) doses at 0.3 mg/kg.
LD50 value, intravenous (mice): 46 mg/kg
LD50 value, subcutaneous (guinea pigs):16 mg/kg.
Fire Hazard
Flash point data for LSD are not available; however LSD is probably combustible.
Safety Profile
Poison by ingestion, subcutaneous, intraperitoneal, and intravenous routes. Mutation data reported. Human systemic effects by ingestion and intramuscular routes: euphoria, hallucinations, distorted perceptions, excitement, anorexia, nausea and vomiting. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. A much-abused hallucinogen. A federally regulated substance. When heated to decomposition it emits toxic fumes of NOx
Environmental Fate
LSD may exist in the air or soil. In the air, LSD may be susceptible to photochemical reactions with subsequently induced radical analogs. Photolysis and oxidative degradation may occur with an airborne half-life of 18 min. LSD’s pKa of 7.8 will have a meaningful percentage of the drug in the cationic form allowing it to interact with soil.
Toxicity evaluation
LSD’s mechanism of action is not completely understood. LSD’s hallucinogenic effects are secondary to its ability to increase central serotonin activity. LSD also stimulates both D1 and D2 dopamine receptors.
Properties of Lysergic acid diethylamide
| Melting point: | 91-93?C |
| Boiling point: | 461.9°C (rough estimate) |
| alpha | D20 +17° (c = 0.5 in pyridine) |
| Density | 1.1021 (rough estimate) |
| refractive index | 1.6200 (estimate) |
| Flash point: | 2℃ |
| storage temp. | −20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly, Heated), Pyridine (S |
| form | Solid |
| pka | pKa 7.5 (Uncertain) |
| color | Pale Brown to Brown |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | Lysergide (50-37-3) |
Safety information for Lysergic acid diethylamide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P337+P313:IF eye irritation persists: Get medical advice/attention. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Lysergic acid diethylamide
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
