LX-1606 Hippurate
- CAS NO.:1033805-22-9
- Empirical Formula: C27H26ClF3N6O3
- Molecular Weight: 574.98
- MDL number: MFCD18251583
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
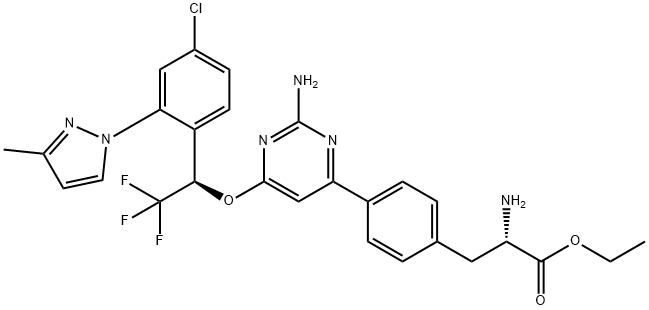
What is LX-1606 Hippurate?
Absorption
After a single oral dose of telotristat ethyl to healthy subjects, telotristat ethyl was absorbed and metabolized to its active metabolite, telotristat. Peak plasma concentrations of telotristat ethyl were achieved within 0.5 to 2 hours, and those of telotristat within 1 to 3 hours. Plasma concentrations thereafter declined in a biphasic manner. Following administration of a single 500 mg dose of telotristat ethyl (twice the recommended dosage) under fasted conditions in healthy subjects, the mean Cmax and AUC0-inf were 4.4 ng/mL and 6.23 ng?hr/mL, respectively for telotristat ethyl. The mean Cmax and AUC0-inf were 610 ng/mL and 2320 ng?hr/mL, respectively for telotristat. Peak plasma concentrations and AUC of telotristat ethyl and telotristat appeared to be dose proportional following administration of a single dose of telotristat ethyl in the range of 100 mg (0.4 times the lowest recommended dose to 1000 mg [4 times the highest recommended dose]) under fasted conditions.
Following multiple-dose administration of telotristat ethyl 500 mg three times daily, there was negligible accumulation at steady state for both telotristat ethyl and telotristat.
In patients with metastatic neuroendocrine tumors and carcinoid syndrome diarrhea treated with SSA therapy, the median Tmax for telotristat ethyl and telotristat was approximately 1 and 2 hours, respectively. Following administration of 500 mg telotristat ethyl three times daily, with meals in patients, the mean Cmax and AUC0-6hr were approximately 7 ng/mL and 22 ng?hr/mL, respectively, for telotristat ethyl. The mean Cmax and AUC0-6hr were approximately 900 ng/mL and 3000 ng?hr/mL, respectively for telotristat. The pharmacokinetic parameters for both telotristat ethyl and telotristat were highly variable with about 55% coefficient of variation.
Toxicity
An embryo-fetal development study performed in rats with oral telotristat ethyl at doses up to 750 mg/kg/day (approximately 9 times the AUC [area under the plasma concentration-time curve] for the active metabolite at the RHD) during organogenesis produced no harm to embryo-fetal development.
In pregnant rabbits treated orally with telotristat ethyl during organogenesis, an increased incidence of post-implantation loss at doses of 250 and 500 mg/kg/day (approximately 15 times the AUC for the active metabolite at RHD) and a decrease in fetal weight at 500 mg/kg/day (approximately 33 times the AUC for the active metabolite at the RHD) was observed. The adverse effects on embryo-fetal development were associated with maternal toxicity (impaired weight gain and/or mortality) at 250 and 500 mg/kg/day. No adverse effects on embryo-fetal development were observed at 125 mg/kg/day (approximately 5 times the AUC for the active
metabolite at the RHD).
A pre-/postnatal development study was conducted in rats using oral administration of 100, 200, and 500 mg/kg/day telotristat ethyl during organogenesis through lactation. An increased incidence of pup mortality was observed during postnatal days 0 to 4 at the maternal dose of 500 mg/kg/day (approximately 5 times the AUC for the active metabolite at the RHD). No developmental abnormalities or effects on growth, learning, memory or reproductive performance were observed through the maturation of offspring at maternal doses of up to 500 mg/kg/day in surviving offspring.
In a 26-week study in transgenic (Tg.rasH2) mice, telotristat ethyl was not tumorigenic at oral doses up to 300 mg/kg/day (approximately 12 to 19 times the AUC for the active metabolite at the RHD).
In a 2-year carcinogenicity study in Sprague-Dawley rats, telotristat ethyl was not tumorigenic at oral doses up to 170 mg/kg/day (approximately 2 to 5 times the AUC for the active metabolite at the RHD).
Telotristat ethyl was negative in the in vitro Ames test, the in vitro chromosomal aberration test using Chinese hamster ovary cells, and the in vivo rat micronucleus test.
Telotristat ethyl at oral doses up to 500 mg/kg/day (approximately 5 times the AUC for the active metabolite at the RHD) was found to have no effect on the fertility and reproductive performance of male or female rats.
The Uses of LX-1606 Hippurate
Telotristat Ethyl can be used in biological study of serotonin biosynthesis as a predictive marker of serotonin pharmacodynamics and disease-induced dysregulation.
Indications
Xermelo is indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy.
Background
Telotristat ethyl is a prodrug of telotristat that was approved by the FDA in March 2017 as Xermelo. It was previously referred to as telotristat etiprate, the hippurate salt form; however, the FDA recommends the use of the name of the neutral form rather than that of the salt. Currently, telotristat ethyl is used to treat carcinoid syndrome diarrhea from neuroendocrine tumors that are inadequately controlled by short-acting somatostatin analog (SSA) treatment.
Neuroendocrine cells are cells that secrete regulatory peptides and biogenic amines in response to chemical, neural, or other types of stimuli. Neuroendocrine tumors (NET) arising from these cells can therefore secrete chemical mediators into the bloodstream to cause side effects in distant sites, a phenomenon called carcinoid syndrome. The most common peptides and amines secreted by NET are histamines, tachykinins, kallikrein, and serotonin. Overexposure to serotonin can cause severe diarrhea, one of the main clinical symptoms of carcinoid syndrome. Serotonin is metabolized in the urinary metabolite 5-hydroxy indole acetic acid (u5-HIAA), and high levels of u5-HIAA is associated with poor survival outcome in patients with NET. The first line treatment of carcinoid syndrome diarrhea is SSA, but symptoms still reoccur over the course of the disease.
Pharmacokinetics
In normal mice, telotristat etiprate (administered once daily for 4 days at doses of 15–300 mg/kg/day) was found to reduce serotonin levels throughout the gastrointestinal tract. These reductions occurred in a dose dependent fashion with maximal effects observed with doses of telotristat etiprate ≥150 mg/kg. No significant change in brain serotonin or 5-hydroxyindoleacetic acid (5-HIAA, a serotonin metabolite) was observed. Similar findings were seen in Sprague-Dawley rats. Gastrointestinal motility studies were conducted in rats using the charcoal meal test. There was a significant dose-related delay in both gastrointestinal transit and gastric emptying, associated with a reduction in blood serotonin levels and proximal colon serotonin.
A quantitative whole-body autoradiography study was conducted to assess the absorption, distribution and excretion of radioactivity in rats following a single oral dose of telotristat etiprate labeled with carbon 14. Rats were administered either 30 mg/kg or 100 mg/kg of the compound. The distribution of radioactivity was limited to tissues of the hepatic and renal system and the contents of the GI tract. There was no measurable radioactivity in the brain at any dose tested.
Metabolism
After oral administration, telotristat ethyl undergoes hydrolysis via carboxylesterases to telotristat, its active metabolite. Telotristat is further metabolized. Among the metabolites of telotristat, the systemic exposure to an acid metabolite of oxidative deaminated decarboxylated telotristat was about 35% of that of telotristat. In vitro data suggest that telotristat ethyl and telotristat are not substrates for CYP enzymes.
Properties of LX-1606 Hippurate
| Melting point: | 80-82°C |
| Boiling point: | 704.7±70.0 °C(Predicted) |
| Density | 1.42 |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 6.72±0.33(Predicted) |
| color | White to Off-White |
Safety information for LX-1606 Hippurate
Computed Descriptors for LX-1606 Hippurate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8

![(2S)-2-aMino-3-[4-[2-aMino-6-[(1R)-1-[4-chloro-2-(3-Methylpyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]pyriMidin-4-yl]phenyl]propanoic acid, Telotristat](https://img.chemicalbook.in/CAS/20150408/GIF/1033805-28-5.gif)