Lumefantrine
Synonym(s):(9Z)-2,7-Dichloro-9-[(4-chlorophenyl)methylene]-α-[(dibutylamino)methyl]-9H-fluorene-4-methanol;Benflumetol;CPG-56695;Lumefantrine
- CAS NO.:82186-77-4
- Empirical Formula: C30H32Cl3NO
- Molecular Weight: 528.94
- MDL number: MFCD05662268
- EINECS: 617-303-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
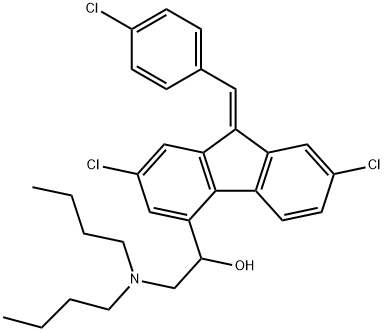
What is Lumefantrine?
Absorption
Food increases absorption.
Toxicity
Common side effects of combination artemether/lumefantrine therapy in adults include headache, anorexia, dizziness, and asthenia. Common side effects in children include pyrexia, cough, vomiting, anorexia, and headache. Possible serious adverse effects include QT prolongation, bullous eruption, urticaria, splenomegaly (9%), hepatomegaly (adults, 9%; children, 6%), hypersensitivty reaction, and angioedema.
Description
Lumefantrine is a derivative of halofantrine that has been reported to exhibit antimalarial activity when combined with artemether in the treatment of multidrug-resistant Plasmodium falciparium . No evidence of cardiotoxicity has been reported with this combination, which may offer promise for successful treatment of resistant organisms.
Description
Lumefantrine is an antimalarial drug that is used in combination with artemether . The pairing of lumefantrine and artemether is a major form of oral artemisinin combination therapy used against uncomplicated P. falciparum malaria. Lumefantrine also blocks the rapidly activating delayed-rectifier potassium channel (IKr; IC50 = 8.1 μM).
Chemical properties
Yellow Solid
The Uses of Lumefantrine
A drug used in the treatment of malaria. Antimalarials are usually classified on the basis of their action against Plasmodia at different stages in their life cycle in the human.
The Uses of Lumefantrine
Lumefantrine has been used:
to study its effect on ex-vivo?Plasmodium falciparum?sensitivity using the tritiated hypoxanthine-based assay
as a standard in the quantification of combined tablet formulation using HPTLC
as a drug molecule in in vitro growth inhibition assay for in vitro B. caballi?growth inhibition studies
The Uses of Lumefantrine
Inhibits hemozoin formation. Antimalarial.
What are the applications of Application
Lumefantrine is an antimalarial which inhibits hemozoin formation
Indications
Lumefantrine and artemether combination therapy is indicated for the treatment of acute uncomplicated malaria caused by Plasmodium falciparum, including malaria acquired in chloroquine-resistant areas. May also be used to treat uncomplicated malaria when the Plasmodium species has not been identified. Indicated for use in adults and children greater than 5 kg.
Background
Lumefantrine is an antimalarial agent used to treat acute uncomplicated malaria. It is administered in combination with artemether for improved efficacy. This combination therapy exerts its effects against the erythrocytic stages of Plasmodium spp. and may be used to treat infections caused by P. falciparum and unidentified Plasmodium species, including infections acquired in chloroquine-resistant areas.
Definition
ChEBI: Lumefantrine is an antimalarial drug used in combination with artemether for the treatment of multi-drug resistant strains of falciparum malaria.
Antimicrobial activity
Lumefantrine has marked blood schizonticidal activity against a wide range of plasmodia, including chloroquineresistant P. falciparum. The 50% and 90% effective concentrations (EC50 and EC90) in vitro are similar: <10 and 40 nmol/L, respectively. The racemate and the two enantiomers exhibit similar activities. Blood schizonticidal activity of desbutylbenflumetol is four to five times greater than benflumetol in vitro.
Acquired resistance
Treatment with artemether–lumefantrine can select for polymorphisms in the P. falciparum pfmdr1 gene. Resistance has been selected experimentally in murine malaria.
General Description
Lumefantrine was developed in China. Itsmechanism of action is poorly understood. There is some evidencethat it inhibits the formation of β-hematin by forming acomplex with hemin. Lumefantrine is very lipophilic and is marketed in combination with the lipophilic artemesininderivedartemether.
Pharmaceutical Applications
A dichlorobenzylidene derivative given orally in combination with artemether.
Biochem/physiol Actions
Lumefantrine is is an antimalarial for the treatment of multi-drug resistant strains of falciparum malaria.
Pharmacokinetics
Bioavailability after oral administration is variable; absorption is substantially increased by co-administration with food, particularly with a high fat content. Peak plasma concentrations occur after 6–8 h. The elimination half-life is 4–6 days. It is almost completely protein bound and metabolized mainly in the liver by CYP3A4.
Pharmacokinetics
Lumefantrine is a blood schizonticide active against erythrocytic stages of Plasmodium falciparum. It is thought that administration of lumefantrine with artemether results in cooperate antimalarial clearing effects. Artemether has a rapid onset of action and is rapidly cleared from the body. It is thus thought to provide rapid symptomatic relief by reducing the number of malarial parasites. Lumefantrine has a much longer half life and is believed to clear residual parasites.
Clinical Use
Treatment of P. falciparum infections (including mixed infections) in a fixed-dose combination treatment with artemether.
Side Effects
The most common adverse effects in combination with artemether include headache, dizziness and gastrointestinal disturbances.
Metabolism
Extensively metabolized in the liver primarily by cytochrome P450 3A4. The major metabolite found in plasma is desbutyl-lumefantrine.
Metabolism
Primaquine is almost totally metabolized by CYP3A4 (99%), with the primary metabolite being carboxyprimaquine. Trace amounts of N-acetylprimaquine plus aromatic hydroxylation and conjugation metabolites also have been reported.
Properties of Lumefantrine
| Melting point: | 129-131°C |
| Boiling point: | 642.5±55.0 °C(Predicted) |
| Density | 1.252 |
| storage temp. | 15-25°C |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| pka | 13.44±0.20(Predicted) |
| color | yellow |
| Merck | 14,5597 |
| CAS DataBase Reference | 82186-77-4(CAS DataBase Reference) |
Safety information for Lumefantrine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lumefantrine
Lumefantrine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 Lumefantrine 99%View Details
Lumefantrine 99%View Details -
 Lumefantrine 98%View Details
Lumefantrine 98%View Details
82186-77-4 -
 Lumefantrine 99%View Details
Lumefantrine 99%View Details -
 82186-77-4 LUMEFANTRINE 95-99%View Details
82186-77-4 LUMEFANTRINE 95-99%View Details
82186-77-4 -
 Lumefantrine 98% CAS 82186-77-4View Details
Lumefantrine 98% CAS 82186-77-4View Details
82186-77-4 -
 Lumefantrine CAS 82186-77-4View Details
Lumefantrine CAS 82186-77-4View Details
82186-77-4 -
 Lumefantrine API PowderView Details
Lumefantrine API PowderView Details
82186-77-4 -
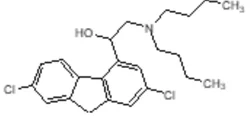 Lumefantrine Stage 2View Details
Lumefantrine Stage 2View Details
69759-61-1
